Abstract
Patients with chronic lymphocytic leukemia (CLL) show suboptimal responses to the vaccines against SARS-CoV-2; it has been shown though that a booster dose of the BNT162b2 vaccine may lead to a significant increase in the seroconversion rates of immunocompromised patients. We conducted a prospective, non-interventional study to evaluate the immunogenicity of a third dose of the BNT162b2 vaccine in adult patients with CLL. Sera were tested before the first, after the second, and before and after the third dose for anti-SARS-CoV-2 receptor binding domain (RBD) spike protein IgG (anti-RBD). Thirty-nine patients with CLL were included in the study. The seroconversion rate increased from 28.2% before the third dose to 64.1% after the third dose and was higher in treatment-naïve patients (72.7% versus 47.1% in actively treated patients, p = 0.042). All but one patient achieving a seroconversion after the second dose retained after the third, while eight patients not achieving a seroconversion after the second dose (38.1%), did so after the third. Moreover, patients actively treated with venetoclax had a higher seroconversion rate than those treated with ibrutinib (87.5% versus 14.3%, p = 0.001). This study confirms the beneficial effect of a third dose of the BNT162b2 vaccine on the seroconversion rate in patients with CLL. Our results also strongly suggest that the use of venetoclax is correlated with higher immunogenicity/seroconversion rates than that of ibrutinib, a finding that has been reported by another study. A treatment strategy change during the pandemic favoring the use of venetoclax may be suggested based on our results, although these results should be validated in larger studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic lymphocytic leukemia (CLL) are vulnerable to severe infection for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with high mortality rates [1,2,3,4,5]. Given the immune deregulation inherent to CLL but also aggravated by the treatment, response of patients with CLL to COVID-19 vaccines has been shown to be suboptimal [6,7,8,9,10,11]. Hence, strategies optimizing response to SARS-CoV-2 vaccination is crucial in these patients. Among the available strategies, a booster dose of the vaccine has been proven effective in immunocompetent individuals since trials have shown that a third dose of the BNT162b2 COVID-19 mRNA vaccine increases the antibody levels against the spike protein of SARS-CoV-2 [12,13,14,15].
Additionally, in studies of solid-organ (mainly kidney) transplant recipients, following a third mRNA vaccine dose, seroconversion has been observed in rates ranging from 30 to 49% of previously seronegative patients that received the standard two-dose regimen [16,17,18,19], while similarly designed studies have provided conflicting results in patients with CLL [20, 21].
In an in-press study from our group, we showed that patients with CLL have suboptimal response to the BNT162b2 vaccine [11]. In the present study, we assessed the immunogenicity of a third dose of the BNT162b2 COVID-19 vaccine in patients with CLL.
Methods
Patients
This is an extension of a previous study on the immunogenicity and safety of two doses of the BNT162b2 mRNA COVID-19 vaccine in patients with CLL [11]. Adult patients with CLL from three tertiary hospitals in Athens, Greece, vaccinated against SARS-CoV-2 with three doses of the BNT162b2 mRNA COVID-19 vaccine participated in the present study after providing a written informed consent. All patients had participated in the first phase of the study with the first two doses of the vaccine. Exclusion criteria included known human immunodeficiency virus infection, vaccination with other anti-SARS-CoV2 vaccines, and inability to provide written informed consent. All patients were approached by the treating physicians and enrolled in a consecutive manner. The study started on October 12, 2021, and its duration was 6 months. At baseline, the epidemiological, clinical, and laboratory characteristics of the patients as well as treatment data were recorded as follows. Age and disease stage at the time of vaccination, disease duration, complete blood count parameters (hemoglobin level, lymphocyte, neutrophil, monocyte, and platelet count), and gamma-globulin levels were recorded and analyzed. Moreover, data on the treatment of the patients (treatment lines, previous treatment with anti-CD20 antibodies, fludarabine, ibrutinib, or venetoclax, active treatment, and treatment regimen at the time of vaccination) were also collected and analyzed.
Vaccination
Patients were vaccinated with the third 30-mcg dose of the BNT162b2 mRNA COVID-19 vaccine administered intramuscularly in the deltoid muscle, according to the national program for vaccination against SARS-CoV-2. The time interval between the second and the third dose of the vaccine was also recorded.
Study procedures
This is a prospective non-interventional study designed to assess immunogenicity against SARS-CoV-2 at baseline (i.e., within 5 days before the third dose of the vaccine) and within 12–21 days after the third dose of the vaccine. Blood samples were collected at the predefined time points and sera were obtained after centrifugation and stored at − 80 °C.
Sera were tested for anti-SARS-CoV-2 receptor-binding domain (RBD) spike protein IgG (anti-RBD), using the Abbott SARS-CoV-2 IgG II Quant assay (Abbott Laboratories, Abbott Park, IL, USA), a two-step chemiluminescent microparticle immunoassay for the qualitative/quantitative detection of IgG antibodies against the RBD of the S1 subunit of the spike protein in human serum and plasma on the Architect i system. The details of the method and its clinical sensitivity and specificity have been provided elsewhere [11, 22, 23]. The assay threshold of 50 AU/mL was set as the seroconversion cutoff in the present study.
The study was approved by the Institutional Review Boards of all three participating centers (Laikon General Hospital, Athens, Greece, 01/22/2021, Attikon Hospital, Athens, Greece 02/24/2021, G. Gennimatas General Hospital, Athens, Greece, 02/18/2021).
Statistical analysis
The IBM SPSS statistics, version 26 (IBM Corporation, North Castle, NY, USA), was used for the statistical analysis of the results. The Pearson chi-square test was used to test for associations between categorical variables, and the Fisher’s exact test was used instead for associations with less than five values in each category. The independent-samples Mann–Whitney U test was used for testing between a categorical variable with two levels and not normally distributed continuous variables, and the Kruskal–Wallis H test for categorical variables with more than two levels. The level of significance for all statistical tests was set at a probability value lower than 5% (2-sided p < 0.05).
Results
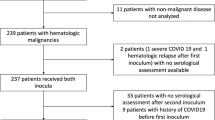
Among 61 patients with CLL participating in the first study, thirty-nine patients vaccinated with the third dose of the BNT162b2 mRNA COVID-19 vaccine were included in the present analysis. The remaining 22 patients either refused to get vaccinated with the third dose (N = 5) or were vaccinated with another vaccine (N = 11) or were not vaccinated with the third dose during the predefined 6-month duration of the study (N = 6). All patients had measurements of the antibody titers before the first and after the second dose of the vaccine, as well as before and after the third dose of the vaccine. The main epidemiologic, clinical, laboratory, and treatment characteristics of the patients are shown in Table 1.
Pre-3rd dose samples were obtained within a median time of 2 (range, 0–5) days before the third dose of the vaccine, while post-3rd dose samples were obtained within a median time of 14 (range, 12–19) days after the third dose of the vaccine.
The median time between the second and third dose of the vaccine was 5.6 (range, 2.0–7.7) months.
Immunogenicity/seroconversion results
The median post-2nd dose antibody titer was 28.6 AU/mL (0.0–40,000) with 18 patients (46.2%) achieving seroconversion, while the median pre-3rd dose antibody titer was 10.5 AU/mL (0.0–7869.2) with the seroconversion rate declining to 28.2% (11 patients). Out of 18 patients who achieved seroconversion with the first two doses of the vaccine, 10 (55.5%) retained this status before the 3rd dose, while out of 21 patients not seroconverted after the first two doses, seroconversion was detected in only one before the third dose, implying a natural infection during the time interval between the two samples collections, although there was no report of COVID19 in any of the patients.
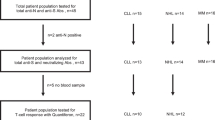
The median post-3rd dose antibody titer was 390.5 AU/mL (1.1–39,264.7) with 25 patients (64.1%) achieving seroconversion (Fig. 1, Table 2). The seroconversion rate was not correlated with any of the studied baseline characteristics of the patients (age, gender, RAI stage, hemoglobin and gamma-globulin level, lymphocyte and platelet count), while treatment-naïve patients had a higher seroconversion rate (16/22, 72.7%) than actively treated patients (8/17, 47.1%), p = 0.042.
Among 21 patients not achieving seroconversion with the first two doses of the vaccine, 8 (38.1%) achieved seroconversion after the third dose, while only one patient, who achieved seroconversion with the first two doses, lost it after the third. This was a 65-year-old treatment-naïve female with worsening hypogammaglobulinemia.
In patients who achieved seroconversion after the 2nd dose of the vaccine and retained it after the 3rd dose (N = 17), there was a non-statistically significant increase in the antibody titer (10,593.6 AU/mL versus 6981.0 AU/mL, p = 0.109). Since the study was not designed to record COVID-19, but only immunogenicity results, there was no active monitoring for COVID-19 in the study population. The median follow-up of the patients since the first sample collection was 7.3 months (range 3.8–11.1).
Among 17 actively treated patients, seroconversion was achieved in 8 (44.4%). Fifteen out of 17 actively treated patients were treated with either ibrutinib (N = 7; median duration of treatment, 16.5 months; range 5–45 months) or venetoclax monotherapy (N = 8; median duration of treatment 29, months; range 15–29 months). Among those patients, there was a statistically significant difference of seroconversion after the 3rd dose (1/7 ibrutinib treated patients versus 7/8 venetoclax treated patients achieved seroconversion, p = 0.005), with a corresponding statistically significant difference in the antibody titers between the two groups (10.7 AU/mL versus 14,989.2 AU/mL respectively, p = 0.001, Fig. 2, Table 2). Ibrutinib- and venetoclax-treated patients did not differ in terms of RAI stage (p = 0.813), hemoglobin level (p = 0.980), lymphocyte (p = 0.132) or platelet count (p = 0.315), gamma globulin levels (p = 0.315), or treatment duration (p = 0.592).
Discussion
Administering a booster dose of a vaccine is a common practice, after initial immunization [24,25,26,27], to provide a re-exposure to the immunizing antigen and consequently increase immunity against the antigen. The need for a booster dose of the vaccines against SARS-CoV-2 was evidenced by studies on the kinetics of the antibodies against the spike protein of the virus, showing that they declined after several months from the first exposure to the antigen [28,29,30,31]. Thus, a booster dose for all available vaccines against SARS-CoV-2 became a priority for immunocompetent and immunocompromised hosts. The well-established results of the booster dose of the BNT162b2 mRNA COIVD-19 vaccine in the general population [32,33,34] have been challenged in patients with immunosuppression, such as malignancies, transplantation, or hematologic diseases, and several studies have tried to assess the immunogenicity and efficacy of the third dose of the vaccine in these populations.
In September 2021, encouraging results from a study in 160 kidney transplant recipients and 20 patients with CLL vaccinated with a third dose of an mRNA COVID-19 vaccine showed that the antibody levels were moderately increased; nevertheless, this increase might not be clinically significant since few patients reached a threshold associated with vaccine effectiveness [35]. Another study showed that 23.8% of 172 patients with CLL who failed to respond to the first two doses of the BNT162b2 mRNA COIVD-19 vaccine responded to a third dose [20]. On the contrary, in another study in patients with several hematologic malignancies, none of the 15 evaluated patients with CLL responded to the third dose of the vaccine [21].
In our study, 38.1% of patients not achieving seroconversion after the second dose of the BNT162b2 mRNA COVID-19 vaccine achieved seroconversion after the third dose. Moreover, all but one patient (94.4%), who achieved seroconversion after the second dose, retained it after the booster dose, with a non-statistically significant trend for higher antibody titers. These results along with previously published studies justify the administration of a booster dose in patients with CLL and probably encourage the administration of a fourth dose in these patients.
In accordance with previous studies, treatment-naïve patients had higher seroconversion rates than actively treated patients [20, 36, 37]. A clinically significant result in our study was the noticeable difference in the seroconversion rate among patients under treatment with ibrutinib and venetoclax, favoring the latter. This result was also highlighted in the results of the first phase of the study after the first two doses of the vaccine, but in the present study is even more pronounced and in accordance with the results of a recent study that showed that patients under venetoclax monotherapy achieved significantly higher response rates than those treated with Bruton tyrosine kinase inhibitor (BTKi) or BTKi in combination with anti-CD20 monoclonal antibodies or venetoclax [38]. This finding, until otherwise proven, may justify a temporary shift in the treatment paradigm for patients with CLL, favoring the administration of venetoclax over BTKi, during the pandemic, given that the data is really strong, but also taking into consideration the small number of patients included in the analysis.
The strengths of the present study are the inclusion of a homogeneous population of patients with CLL vaccinated with only one type of anti-SARS-CoV-2 vaccine, as well as the analysis of patients treated with ibrutinib and venetoclax monotherapy that yielded a decision-making result that may affect treatment selection in patients with CLL. The main limitation of this study is the small patient sample that did not permit further statistical analyses. The small patient sample may also explain the lack of correlation of seroconversion rate with the baseline characteristics of the patients.
Finally, an inherent limitation of the study is the lack of detection of cellular immune responses in the studied population. It has been reported by several authors that the impaired humoral response in patients with CLL vaccinated against SARS-CoV-2 may be counteracted by an effective cellular response. Thus, it has been shown that even though serological response is severely impaired in patients with CLL especially when they are treated with rituximab or ibrutinib, a significant proportion of the patients may develop sufficient cell-mediated immunity [39]. Moreover, although early cellular immune responses rely mostly on CD8 + T cells and these cells are significantly increased after vaccination, they still remain lower than normal, and this may play a role in the reduced memory response and the need for booster doses, especially in immunocompromised hosts such as patients with CLL [40]. On the other hand, there are reports of unaffected numbers of specific CD8 + T cells against SARS-CoV-2 after vaccination in patients with CLL [41].
In conclusion, patients with CLL may benefit from a booster dose of the BNT162b2 mRNA COVID-19 vaccine, since more than a third of patients not achieving seroconversion with the first two doses, seroconverted after the third. Moreover, venetoclax monotherapy seems to be a valuable treatment choice for patients with CLL during the pandemic for yet another reason, apart from its efficacy and favorable safety profile; the high rates of seroconversion after vaccination against SARS-CoV-2.
Data availability
Data on the experiments and patient data are available upon reasonable request. For original data, please contact pandiamantopoulos@gmail.com.
References
Parry H, McIlroy G, Bruton R, Damery S, Tyson G, Logan N et al (2022) Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol 15(1):3
Muntañola A, Villacampa G, Hernández-Rivas J, Alonso R, Mirás F, Osorio S et al (2020) Clinical characteristics and outcome of SARS-CoV-2 infection in admitted patients with chronic lymphocytic leukemia from a single European country. Exp Hematol Oncol 9(1):37
Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C et al (2020) COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 34(9):2354–2363
Jin XH, Zheng KI, Pan KH, Xie YP, Zheng MH (2020) COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol 7(4):e351–e352
Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M et al (2020) Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol 190(1):e16–e20
Mellinghoff SC, Robrecht S, Mayer L, Weskamm LM, Dahlke C, Gruell H et al (2022) SARS-CoV-2 specific cellular response following COVID-19 vaccination in patients with chronic lymphocytic leukemia. Leukemia 36(2):562–565
Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N et al (2022) Safety and efficacy of the BNT162b mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Haematologica 107(3):625–634
Molica S, Giannarelli D, Lentini M, Zappala D, Mannella A, Loiacono D et al (2021) Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia: a serologic and cellular study. Chemotherapy 67(2):91–95
Fox TA, Kirkwood AA, Enfield L, O’Reilly M, Arulogun S, D’Sa S et al (2021) Low seropositivity and suboptimal neutralisation rates in patients fully vaccinated against COVID-19 with B-cell malignancies. Br J Haematol 195(5):706–709
Shen Y, Freeman JA, Holland J, Solterbeck A, Naidu K, Soosapilla A et al (2021) COVID-19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B-lymphocytosis; humoural and cellular immunity. Br J Haematol 197(1):41–51
Diamantopoulos P, Stafilidis SC, Vlachopoulou D, Kontandreopoulou C-N, Giannakopoulou N, Vardaka M et al (2022) Safety and immunogenicity of the BNT162b2 mRNA Covid-19 vaccine in patients with chronic lymphocytic leukemia. A prospective study. Ther Adv Hematol 13:20406207221090150
Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C et al (2022) mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 28(3):477–480
Chenchula S, Karunakaran P, Sharma S, Chavan M (2022) Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol 94(7):2969–2976
Butt AA, Talisa VB, Yan P, Shaikh OS, Omer SB, Mayr FB (2022) Vaccine effectiveness of three vs. two doses of SARS-CoV-2 mRNA vaccines in a high risk national population. Clin Infect Dis 75(1):e579–e584
Romero-Ibarguengoitia ME, Rivera-Salinas D, Hernández-Ruíz YG, Armendariz-Vázquez AG, González-Cantú A, Barco-Flores IA et al (2022) Effect of the third dose of BNT162b2 vaccine on quantitative SARS-CoV-2 spike 1–2 IgG antibody titers in healthcare personnel. PLoS One 17(3):e0263942
Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM et al (2021) Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 174(9):1330–1332
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A (2021) Three doses of an mRNA Covid-19 Vaccine in solid-organ transplant recipients. N Engl J Med 385(7):661–662
Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S et al (2021) Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 326(11):1063–1065
Charmetant X, Espi M, Benotmane I, Barateau V, Heibel F, Buron F et al (2022) Infection or a third dose of mRNA vaccine elicit neutralizing antibody responses against SARS-CoV-2 in kidney transplant recipients. Sci Transl Med 14(636):eabl6141
Herishanu Y, Rahav G, Levi S, Braester A, Itchaki G, Bairey O et al (2022) Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood 139(5):678–685
Šušol O, Hájková B, Zelená H, Hájek R (2022) Third dose of COVID-19 vaccine restores immune response in patients with haematological malignancies after loss of protective antibody titres. Br J Haematol 197(3):302–305
Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A et al (2020) Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 58(8):e00941–20
Available from: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2-. Accessed 6 Aug 2022
(2011) Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics. 128(6):1213–1218
Palgen JL, Feraoun Y, Dzangué-Tchoupou G, Joly C, Martinon F, Le Grand R et al (2021) Optimize Prime/boost vaccine strategies: trained immunity as a new player in the game. Front Immunol 12:612747
Gilca V, Sauvageau C, Boulianne N, De Serres G, Crajden M, Ouakki M et al (2015) The effect of a booster dose of quadrivalent or bivalent HPV vaccine when administered to girls previously vaccinated with two doses of quadrivalent HPV vaccine. Hum Vaccin Immunother 11(3):732–738
Knuf M, Habermehl P, Faber J, Bock HL, Sänger R, Bogaerts H et al (2006) Assessment of nine candidate DTP-vaccines with reduced amount of antigen and/or without adjuvant as a fourth (booster-) dose in the second year of life. Vaccine 24(27–28):5627–5636
Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM et al (2020) A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 11(1):4704
Bläckberg A, Fernström N, Sarbrant E, Rasmussen M, Sunnerhagen T (2021) Antibody kinetics and clinical course of COVID-19 a prospective observational study. PLoS One 16(3):e0248918
Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS et al (2021) Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med 385(21):2010–2012
Barouch DH, Stephenson KE, Sadoff J, Yu J, Chang A, Gebre M et al (2021) Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 385(10):951–3
Spitzer A, Angel Y, Marudi O, Zeltser D, Saiag E, Goldshmidt H et al (2022) Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA 327(4):341–349
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N et al (2021) Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 385(15):1393–1400
Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK et al (2022) Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am 9:100198
Marlet J, Gatault P, Maakaroun Z, Longuet H, Stefic K, Handala L et al (2021) Antibody responses after a third dose of COVID-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines (Basel) 9(10):1055
Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N et al (2021) COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia 35(9):2703–2705
Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S et al (2021) Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 11(7):136
Bagacean C, Letestu R, Al-Nawakil C, Brichler S, Lévy V, Sritharan N et al (2022) Humoral response to mRNA anti-COVID-19 vaccines BNT162b2 and mRNA-1273 in patients with chronic lymphocytic leukemia. Blood Adv 6(1):207–211
Bacova B, Kohutova Z, Zubata I, Gaherova L, Kucera P, Heizer T et al (2022) Cellular and humoral immune response to SARS-CoV-2 mRNA vaccines in patients treated with either Ibrutinib or Rituximab. Clin Exp Med 1–9
Rodríguez-Mora S, Corona M, Torres M, Casado-Fernández G, García-Pérez J, Ramos-Martín F et al (2022) Early cellular and humoral responses developed in oncohematological patients after vaccination with one dose against COVID-19. J Clin Med 11(10):2803
Zaleska J, Kwasnik P, Paziewska M, Purkot J, Szabelak A, Jurek M et al (2022) Response to anti-SARS-CoV-2 mRNA vaccines in multiple myeloma and chronic lymphocytic leukemia patients. Int J Cancer. https://doi.org/10.1002/ijc.34209
Acknowledgements
We would like to thank Mr. Zisis Moschidis and Mr. Evangelos Kokolesis for conducting the experiments, Ms. Lina Malakou, Mr. Konstantinos Theodorakopoulos, Ms. Aphroditi Lazarakou, and Mr. Georgios Kyriakakis for sample handling.
Funding
Open access funding provided by HEAL-Link Greece This work was supported by SYN-ENOSIS (protocol number 55/13–10-2020) and donations from SB Bioanalytica SA, Viatris Hellas, and the Hellenic Scientific Society for the Study of AIDS, Sexually Transmitted and Emerging Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experiments were performed in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Boards of all three participating centers (Laikon General Hospital, Athens, Greece, 01/22/2021, Attikon Hospital, Athens, Greece 02/24/2021, G. Gennimatas General Hospital, Athens, Greece, 02/18/2021).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diamantopoulos, P.T., Kontandreopoulou, CN., Stafylidis, C. et al. Immunogenicity of a third dose of the BNT162b2 COVID-19 vaccine in patients with CLL: effects on treatment selection. Ann Hematol 101, 2711–2717 (2022). https://doi.org/10.1007/s00277-022-05003-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-05003-6