Abstract
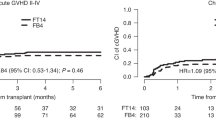
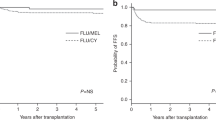
We evaluated 413 adult patients with lymphoma who underwent unrelated cord blood transplantation (UCBT) with fludarabine and melphalan (FM)-based reduced-intensity conditioning between 2002 and 2017 to investigate longitudinal changes in outcomes and the optimal melphalan dose and graft-versus-host disease (GVHD) prophylaxis regimen. Outcomes were compared between FM80/100 (melphalan dose: 80 or 100 mg/m2) and FM140 (melphalan dose: 140 mg/m2), as well as between calcineurin inhibitor (CNI) plus methotrexate (MTX), CNI plus mycophenolate mofetil (MMF), and CNI alone. The 3-year overall survival (OS) and non-relapse mortality (NRM) rates improved over time (OS: 27% in 2000s vs. 42% in 2010s, p < 0.001; NRM: 43% in 2000s vs. 26% in 2010s, p < 0.001). Multivariable analysis showed that in the 2000s, melphalan dose and GVHD prophylaxis regimen did not affect any outcomes. In the 2010s, FM80/100 (vs. FM140) related to better OS (hazard ratio [HR] 0.62, p = 0.01) and NRM (HR 0.52, p = 0.016). MTX + CNI and CNI alone (vs. CNI + MMF) related to worse OS (CNI + MTX, HR 2.01, p < 0.001; CNI alone, HR 2.65, p < 0.001) and relapse/progression (CNI + MTX, HR 2.40, p < 0.001; CNI alone, HR 2.13, p = 0.023). In recent years, the use of FM80/100 and CNI + MMF significantly reduced the risk of NRM and relapse/progression, respectively, and resulted in better OS after UCBT for lymphoma.





Similar content being viewed by others
Data availability
For anonymized clinical data without patient sensitive information, please contact the corresponding author with reasonable request.
References
Ballen KK, Gluckman E, Broxmeyer HE (2013) Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122:491–498
Mayani H, Wagner JE, Broxmeyer HE (2020) Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant 55:48–61
Corradini P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R et al (2007) Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia 21:2316–2323
Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ et al (2008) Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 14:1279–1287
Chen R, Palmer JM, Popplewell L, Shen J, Smith E, Delioukina M et al (2011) Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol 90:803–808
Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO (2013) Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19:344–356
Saini NY, Saliba RM, Rondon G, Maadani F, Popat U, Hosing CM et al (2019) Impact of donor type and melphalan dose on allogeneic transplantation outcomes for patients with lymphoma. Biol Blood Marrow Transplant 25:1340–1346
Danylesko I, Shimoni A, Nagler A (2012) Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant 47:5–14
Cruz JG, Martino R, Balsalobre P, Heras I, Pinana JL, Serrano D et al (2011) Long-term results of fludarabine/melphalan as a reduced-intensity conditioning regimen in mantle cell lymphoma: the GELTAMO experience. Ther Adv Hematol 2:5–10
Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G et al (2004) Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood 104:3865–3871
Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M et al (2006) Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant 12:172–183
Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT et al (2008) Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated MD Anderson Cancer Center experience. Haematologica 93:257–64
Thomson KJ, Morris EC, Bloor A, Cook G, Milligan D, Parker A et al (2009) Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol 27:426–432
Delioukina M, Zain J, Palmer JM, Tsai N, Thomas S, Forman S (2012) Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant 47:65–72
Lee JH, Lee JH, Kim DY, Seol M, Lee YS, Kang YA et al (2015) A phase II trial of fludarabine/melphalan 100 conditioning therapy followed by allogeneic hematopoietic cell transplantation for patients with lymphoma. Clin Lymphoma Myeloma Leuk 15:655–663
Kekre N, Marquez-Malaver FJ, Cabrero M, Pinana J, Esquirol A, Soiffer RJ et al (2016) Fludarabine/busulfan versus fludarabine/melphalan conditioning in patients undergoing reduced-intensity conditioning hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transplant 22:1808–1815
Harada K, Yanada M, Machida S, Kanamori H, Onizuka M, Ozawa Y et al (2019) Prognostic impact of melphalan dose and total body irradiation use in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation with reduced-intensity conditioning. Leuk Lymphoma 60:1493–1502
Bashir Q, Khan H, Thall PF, Liu P, Shah N, Kebriaei P et al (2013) A randomized phase II trial of fludarabine/melphalan 100 versus fludarabine/melphalan 140 followed by allogeneic hematopoietic stem cell transplantation for patients with multiple myeloma. Biol Blood Marrow Transplant 19:1453–1458
Al Malki MM, Nathwani N, Yang D, Armenian S, Dadwal S, Salman J et al (2018) Melphalan-based reduced-intensity conditioning is associated with favorable disease control and acceptable toxicities in patients older than 70 with hematologic malignancies undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 24:1828–1835
Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N et al (2005) Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant 11:314–318
Uchida N, Wake A, Nakano N, Ishiwata K, Takagi S, Tsuji M et al (2011) Mycophenolate and tacrolimus for graft-versus-host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation 92:366–371
Narimatsu H, Terakura S, Matsuo K, Oba T, Uchida T, Iida H et al (2007) Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant 39:31–39
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A et al (2007) Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol 86:269–274
Atsuta Y (2016) Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol 103:3–10
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al (1995) 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825–828
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB et al (1981) Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 57:267–276
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–1633
Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18:695–706
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162:W1-73
Moons KG, Donders RA, Stijnen T, Harrell FE Jr (2006) Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 59:1092–1101
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Chen GL, Hahn T, Wilding GE, Groman A, Hutson A, Zhang Y et al (2019) Reduced-intensity conditioning with fludarabine, melphalan, and total body irradiation for allogeneic hematopoietic cell transplantation: the effect of increasing melphalan dose on underlying disease and toxicity. Biol Blood Marrow Transplant 25:689–698
Xu H, Exner BG, Chilton PM, Tanner MK, Mueller YM, Rezzoug F et al (2004) A delay in bone marrow transplantation after partial conditioning improves engraftment. Transplantation 77:819–826
Cutler C, Ballen K (2009) Reduced-intensity conditioning and umbilical cord blood transplantation in adults. Bone Marrow Transplant 44:667–671
Narimatsu H, Watanabe M, Kohno A, Sugimoto K, Kuwatsuka Y, Uchida T et al (2008) High incidence of graft failure in unrelated cord blood transplantation using a reduced-intensity preparative regimen consisting of fludarabine and melphalan. Bone Marrow Transplant 41:753–756
Horwitz ME, Morris A, Gasparetto C, Sullivan K, Long G, Chute J et al (2008) Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant 14:591–594
Uchida N, Wake A, Takagi S, Yamamoto H, Kato D, Matsuhashi Y et al (2008) Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biol Blood Marrow Transplant 14:583–590
Terakura S, Kuwatsuka Y, Yamasaki S, Wake A, Kanda J, Inamoto Y et al (2017) GvHD prophylaxis after single-unit reduced intensity conditioning cord blood transplantation in adults with acute leukemia. Bone Marrow Transplant 52:1261–1267
Martin PJ (1993) Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med 178:703–712
Fatobene G, Rocha V, St Martin A, Hamadani M, Robinson S, Bashey A et al (2020) Nonmyeloablative alternative donor transplantation for Hodgkin and non-Hodgkin lymphoma: from the LWP-EBMT, Eurocord, and CIBMTR. J Clin Oncol 38:1518–1526
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al (2011) Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 118:282–288
Funding
This work was supported by a grant from the National Cancer Research and Development Fund (2020-A-15).
Author information
Authors and Affiliations
Contributions
K.S. designed the research, analyzed the data, performed the statistical analysis, and wrote the first draft of the manuscript. S.W.K. designed the research and contributed to the critical review of the manuscript. H.O. analyzed the data and performed the statistical analysis. M.K., K.K., and S.Y. contributed to the critical review of the manuscript. All the other authors contributed to data collection. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the data management committee of the Japanese Society for Transplantation and Cellular Therapy and by the institutional review board of Osaka City University (Osaka, Japan). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
The Transplant Registry Unified Management Program (TRUMP) database of the Japanese Society for Transplantation and Cellular Therapy and the Japanese Data Center for Hematopoietic Cell Transplantation includes physician-reviewed data. Observational studies based on the TRUMP database are performed with informed consent.
Competing interests
K.K. received honoraria from MSD K.K., Chugai Pharmaceutical Co., Ltd. N.T. received honoraria from Chugai Pharmaceutical Co., Ltd. and Pfizer Inc., Sanofi S.A., and research funding from Chugai Pharmaceutical Co., Ltd. The remaining authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakatoku, K., Kim, SW., Okamura, H. et al. Improved survival after single-unit cord blood transplantation using fludarabine and melphalan-based reduced-intensity conditioning for malignant lymphoma: impact of melphalan dose and graft-versus-host disease prophylaxis with mycophenolate mofetil. Ann Hematol 101, 2743–2757 (2022). https://doi.org/10.1007/s00277-022-04990-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04990-w