Abstract
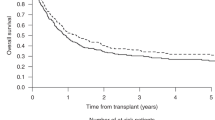
The use of Bcl-2 inhibitor Venetoclax (VEN) combined with hypomethylating agents or chemotherapy has shown efficacy in treating acute myeloid leukemia (AML) as frontline treatment and for relapse, allowing more patients to bridge to allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, the influence of VEN-based therapy on the prognosis of subsequent allogeneic HSCT remains unknown. We retrospectively collected data from patients who proceeded to allo-HSCT between November 2018 and November 2020 after VEN-based therapy at five transplant centers in Zhejiang Province, China. A total of 39 patients were analyzed. Thirty-one patients were diagnosed with AML (28 de novo, 3 secondary to MDS), 6 with MDS, and 2 with CMML. The majority (74.4%) of patients received VEN-based therapy for the treatment of relapse (38.5%) or refractory disease (35.9%); 5 (12.8%) received it as an initial treatment, and 5 (12.8%) patients who were already in complete remission (CR) received VEN for further consolidation or deep remission before HSCT. Twenty-seven (69.2%) patients were in CR at the time of HSCT. Day + 100 cumulative incidences of grade I–IV acute graft-versus-host disease (aGVHD) and grade II–IV aGVHD were 43.6% and 15.4%, respectively. Of 34 evaluable patients, 6.4% and 25.6% developed chronic GVHD at 1 year and 2 years. The 100-day cytomegalovirus (CMV) reactivation occurred in 76.3% of patients and Epstein-Barr virus (EBV) reactivation occurred in 29.7% of patients. With a median follow-up of 14.7 months, overall survival, progression-free survival, relapse, and non-relapse mortality incidence at 1 year were 75.5%, 61.6%, 16.7%, and 21.7%, respectively. Both univariate and multivariate analysis revealed that relapsed/refractory (R/R) disease was associated with inferior PFS (HR 4.849, 95% CI 1.009–23.30; p = 0.049). Prior poor response to VEN was found to be a significant factor predicting higher risk of relapse (HR 4.37, 95% CI 1.130–16.9; p = 0.033). Our results showed that VEN-based regimen therapy followed by allo-HSCT in AML patients is feasible and does not increase the risk of transplant-related mortality and toxicity.



Similar content being viewed by others
References
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT (2013) BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12(3):329–341. https://doi.org/10.1016/j.stem.2012.12.013
Konopleva M, Letai A (2018) BCL-2 inhibition in AML: an unexpected bonus? Blood 132(10):1007–1012. https://doi.org/10.1182/blood-2018-03-828269
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang HC, Humerickhouse RA, Rosenberg SH, Elmore SW (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, Kaiser J, Lyle L, Boggs C, Halsema K, Schowinsky JT, Rosser J, Ewalt MD, Siegele B, Rana V, Schuster S, Abbott D, Stevens BM, Jordan CT, Smith C, Pollyea DA (2019) Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv 3(20):2911–2919. https://doi.org/10.1182/bloodadvances.2019000243
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Dohner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hajek R, Porkka K, Illes A, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW (2020) Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383(7):617–629. https://doi.org/10.1056/NEJMoa2012971
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1):7–17. https://doi.org/10.1182/blood-2018-08-868752
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, Jabbour E, Cortes J, Garcia-Manero G, Ravandi F, Bhalla KN, Kantarjian H, Konopleva M (2018) Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 93(3):401–407. https://doi.org/10.1002/ajh.25000
Zeidan AM, Pollyea DA, Garcia JS, Brunner A, Roncolato F, Borate U, Odenike O, Bajel AR, Watson AM, Gotze K, Nolte F, Tan PT, Hong WJ, Dunbar M, Zhou Y, Gressick L, Ainsworth W, Harb J, Salem AH, Hayslip J, Swords R, Garcia-Manero G (2019) A phase 1b study evaluating the safety and efficacy of venetoclax as monotherapy or in combination with azacitidine for the treatment of relapsed/refractory myelodysplastic syndrome. Blood 134:565. https://doi.org/10.1182/blood-2019-124994
Wei AH, Garcia JS, Borate U, Fong CY, Baer MR, Nolte F, Peterlin P, Jurcic JG, Garcia-Manero G, Hong WJ, Platzbecker U, Odenike O, Dunbar M, Zhou Y, Harb J, Tanwani P, Wolff JE, Jacoby M (2019) A phase 1b study evaluating the safety and efficacy of venetoclax in combination with azacitidine in treatment-naive patients with higher-risk myelodysplastic syndrome. Blood 134:568. https://doi.org/10.1182/blood-2019-124437
Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, Leonard J, Colace SI, Khaw SL, Fleming SA, Mattison RJ, Norris R, Opferman JT, Roberts KG, Zhao Y, Qu C, Badawi M, Schmidt M, Tong B, Pesko JC, Sun Y, Ross JA, Vishwamitra D, Rosenwinkel L, Kim SY, Jacobson A, Mullighan CG, Alexander TB, Stock W (2021) Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov 11(6):1440–1453. https://doi.org/10.1158/2159-8290.CD-20-1465
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374(4):311–322. https://doi.org/10.1056/NEJMoa1513257
Kaufman JL, Gasparetto C, Schjesvold FH, Moreau P, Touzeau C, Facon T, Boise LH, Alzate S, Macartney T, Pesko J, Salem AH, Ross JA, Hong WJ, Maciag PC, Pauff JM, Kumar SK (2019) Phase I/II study evaluating the safety and efficacy of venetoclax in combination with dexamethasone as targeted therapy for patients with t(11;14) relapsed/refractory multiple myeloma. Blood 134:926. https://doi.org/10.1182/blood-2019-125871
Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, Benboubker L, Facon T, Amiot M, Moreau P, Punnoose EA, Alzate S, Dunbar M, Xu T, Agarwal SK, Enschede SH, Leverson JD, Ross JA, Maciag PC, Verdugo M, Touzeau C (2017) Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130(22):2401–2409
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, Xie W, Zheng W, Zhu Y, Ye X, Yu X, Cai Z, Lin M, Huang H (2014) T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 124(17):2735–2743. https://doi.org/10.1182/blood-2014-04-571570
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wolfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE (2016) International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22(1):4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425. https://doi.org/10.1182/blood-2005-10-4149
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis SoRCTO, Reporting Standards for Therapeutic Trials in Acute Myeloid L (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649. https://doi.org/10.1200/JCO.2003.04.036
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, Keating A, Lazarus HM, Litzow MR, Marks DI, Maziarz RT, Rizzieri DA, Schiller G, Schultz KR, Tallman MS, Weisdorf D (2010) Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28(23):3730–3738. https://doi.org/10.1200/JCO.2010.28.8852
Yue X, Chen Q, He J (2020) Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int 20(1):524. https://doi.org/10.1186/s12935-020-01614-z
Aldoss I, Pullarkat V, Stein AS (2021) Venetoclax-containing regimens in acute myeloid leukemia. Ther Adv Hematol 12:2040620720986646. https://doi.org/10.1177/2040620720986646
Sandhu KS, Dadwal S, Yang D, Mei M, Palmer J, Salhotra A, Al Malki M, Aribi A, Ali H, Khaled S, Forman SJ, Snyder D, Nakamura R, Stein AS, Marcucci G, Aldoss I, Pullarkat V (2020) Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant 26(12):e322–e327. https://doi.org/10.1016/j.bbmt.2020.08.027
Pratz KW, DiNardo CD, Arellano ML, Letai AG, Thirman M, Pullarkat VA, Roboz GJ, Becker PS, Hong WJ, Jiang Q, Hayslip J, Potluri J, Pollyea DA (2019) Outcomes after stem cell transplant in older patients with acute myeloid leukemia treated with venetoclax-based therapies. Blood 134:264. https://doi.org/10.1182/blood-2019-127251
Pollyea DA, Winters A, McMahon C, Schwartz M, Jordan CT, Rabinovitch R, Abbott D, Smith CA, Gutman JA (2021) Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant 57(2):160–166. https://doi.org/10.1038/s41409-021-01476-7
Mukherjee A, Milton DR, Jabbour E, Daver N, Gulbis A, Ledesma C, Konopleva M, DiNardo CD, Ravandi F, Kadia TM, Alatrash G, Alousi AM, Daher M, Marin D, Olson AL, Oran B, Kebriaei P, Saini N, Srour SA, Popat UR, Im JS, Mehta R, Rondon G, Kantarjian HM, Champlin RE, Khouri IF (2020) Risk of GvHD and survival in patients with acute leukemia who were bridged to allogeneic stem cell transplantation (alloSCT) with venetoclax-based therapy. Blood 136:13–14. https://doi.org/10.1182/blood-2020-137097
Rausch CR, DiNardo CD, Maiti A, Jammal NJ, Kadia TM, Marx KR, Borthakur G, Savoy JM, Pemmaraju N, DiPippo AJ, Daver NG, Chew SM, Sasaki K, Issa GC, Short NJ, Takahashi K, Ohanian MN, Ning J, Xiao L, Alvarado Y, Kontoyiannis DP, Ravandi F, Kantarjian HM, Konopleva MY (2021) Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer 127(14):2489–2499. https://doi.org/10.1002/cncr.33508
Masarova L, DiNardo CD, Bose P, Pemmaraju N, Daver NG, Kadia TM, Chifotides HT, Zhou L, Borthakur G, Estrov Z, Konopleva M, Verstovsek S (2021) Single-center experience with venetoclax combinations in patients with newly diagnosed and relapsed AML evolving from MPNs. Blood Adv 5(8):2156–2164. https://doi.org/10.1182/bloodadvances.2020003934
Mikulska M, Del Bono V, Viscoli C (2014) Bacterial infections in hematopoietic stem cell transplantation recipients. Curr Opin Hematol 21(6):451–458. https://doi.org/10.1097/MOH.0000000000000088
Vu DL, Dayer JA, Masouridi-Levrat S, Combescure C, Boely E, Khanna N, Mueller NJ, Kleber M, Medinger M, Halter J, Passweg J, Muller AM, Schanz U, Chalandon Y, Neofytos D, van Delden C, Kaiser L, Swiss Transplant Cohort S (2020) Microbiologically documented infections after adult allogeneic hematopoietic cell transplantation: a 5-year analysis within the Swiss Transplant Cohort study. Transpl Infect Dis 22(4):e13289. https://doi.org/10.1111/tid.13289
Styczynski J (2018) Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther 7(1). https://doi.org/10.1007/s40121-017-0180-z
Acknowledgements
The authors acknowledge all collaborating institutions that contributed cases to this study.
Funding
This work was funded by the National Natural Science Foundation of China (grant nos. 82070179, 81970158, and 81970097) and Zhejiang Key R&D Program (Science and Technology Department, grant no. 2020C03G2013586).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by institutional review boards at each study site.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All patients consented to the research and its publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, TT., Song, XL., Zhao, YM. et al. Outcome after allogeneic hematopoietic stem cell transplantation following Venetoclax-based therapy among AML and MDS patients. Ann Hematol 101, 2731–2741 (2022). https://doi.org/10.1007/s00277-022-04983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04983-9