Abstract
Immunosuppressive therapy (IST) is an effective treatment regimen for severe aplastic anaemia (SAA) patients without HLA-identical donors. This study further compared the outcomes between IST and IIST-UCB in SAA on the basis of research shown that IST combined with umbilical cord blood infusion (IIST-UCB) treated effectively. A total of 123 patients from 11 hospitals in China were enrolled. Sixty-nine patients in IIST-UCB group were treated with ATG + CsA + CTX combined with cord blood, while 54 patients in IST group with ATG + CsA. The overall remission rates (ORRs), complete remission (CR) rates and partial response (PR) rates of IIST-UCB group and IST group at 3 months were 69.67% vs 51.85% (P = .045), 21.74% vs 3.7% (P = .004) and 47.83% vs 48.15% (P = .972), respectively. After 6 months of treatment, they were 76.81% vs 57.41% (P = .022), 37.68% vs 11.11% (P = .001) and 39.13% vs 46.30% (P = .425), respectively. After 1 year of treatment, they were 85.51% vs 61.11% (P = .002), 59.42% vs 25.93% (P = .000) and 26.09% vs 35.19% (P = .275), respectively. The ORRs and CR rates of IIST-UCB group were both significantly higher than IST group after 3 months, 6 months and 1 year of treatment. The neutrophil granulocyte, platelet and haemoglobin recovery times of IIST-UCB group were significantly shorter than IST group. Compared with standard IST, IIST-UCB as an effective therapy for SAA patients without HLA-identical donors accelerated the haematopoietic reconstitution, resulting in higher early CR rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Severe aplastic anaemia (SAA) is a bone marrow failure disease characterised by dysregulated haematopoietic stem cell apoptosis and pancytopenia, causing bleeding and infection, which are the main causes of early death [1]. Presently, the treatment for SAA mainly includes allogeneic-haematopoietic stem cell transplantation (allo-HSCT), immunosuppressive therapy (IST) and supportive therapy [2,3,4]. Matching sibling donor haematopoietic stem cell transplantation (MSD-HSCT) is the first-line therapy for children and adolescents with SAA. Unrelated donor HSCT (UD-HSCT) with HLA matching ≥ 8/10 could be an available option [5, 6] for adult and older patients. However, the possibility of finding a matched unrelated donor is rather low and the process is time-consuming and so the SAA patients may miss their best therapeutic opportunity.
IST is composed of antithymocyte globulin and antilymphocyte globulin (ATG/ALG) combined with cyclosporine A (CsA) with an overall effective rate of 60–80%. However, 30% of patients do not respond to IST. Furthermore, the recurrence rate is up to 40%, while the effective rate of these relapsing patients is lower than 50%. In addition, some patients develop clonal diseases, like paroxysmal nocturnal haemoglobinuria and myelodysplastic syndrome, resulting in poorer prognoses [7, 8]. Therefore, it is important to develop new alternative therapies for older SAA patients and patients without HLA-identical donors.
In 1974, Kundtzon [9] found that human umbilical cord blood contained a number of haematopoietic stem/progenitor cells, mesenchymal stem/progenitor cells and cytokines that are able to stimulate haematopoietic reconstitution. Our previous study reported a clinical analysis of 36 cases of children with SAA undergoing intensive immunosuppressive therapy (ATG + CTX) combined with umbilical cord blood transfusions (IIST-UCB). The ORRs after 3 months and 1 year of treatment were 61.1% and 86.2% respectively, and the 3-year OS rate was 83.3%, which showed a better prognosis than patients undergoing standard IST [10]. Hence, we designed this multicentre trial to compare the effects of IST and IIST-UCB on the basis of aforementioned outcome.
Methods
Patients and study design
The trial was conducted from July 2016 to December 2018 with inclusion criteria as follows: (1) SAA and very severe aplastic anaemia (VSAA) patients diagnosed according to Camitta’s criteria [11] without sex or age limitations; (2) patients with newly diagnosed SAA and VSAA did not receive ATG or CSA for more than 6 months within 1 year; (3) no HLA-identical-related donors. The exclusion criteria included (1) congenital aplastic anaemia; (2) pregnancy or lactation; (3) participation in other clinical trials within 3 months before enrolment; (4) life-threatening conditions including respiratory failure, heart failure and liver or kidney failure; (5) aplastic anaemia caused by the treatment of other malignant tumours; (6) severe mental illness; (7) other malignant tumours; (8) serious or unmanageable infections; (9) ATG treatment within 6 months or more than 6 months of CsA treatment; and (10) serious allergies to biological agents. A total of 123 patients were enrolled with 69 received IIST-UCB therapy and 54 received standard IST.
The study was approved by the Ethics Committee of the 960 Hospital of Chinese People’s Liberation Army, and all patients signed informed consent forms. The study was conducted in accordance with the Declaration of Helsinki and has been registered on clinicaltrials.gov (No: NCT02838992).
Treatment protocols
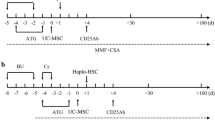
IIST-UCB group received rabbit ATG (r-ATG) 3 mg/kg/day × 5 days (− 6 day to − 2 day), CTX 50 mg/kg/day × 2 days (− 3 day to − 2 day), and infused cord blood haematopoietic stem cells on day 0. CsA (3 mg/kg/day) infusion was started at − 1 day lasting for 12–18 months. The dose of CsA was adjusted according to its blood concentration (maintained at 200–400 ng/mL). The UCB was provided by the Shandong Cord Blood Bank; it was ≥ 4/6 or 5/10 HLA matches and given in a single administration. The UCB was infused into the peripheral blood with number of 4.03 (1.64–13.69) × 107/kg.
IST group received r-ATG 3 mg/kg/day × 5 days (− 5 day to − 1 day). An infusion of 3 mg/kg/day CsA was started at − 1 day lasting for 12–18 months. The dose of CsA was adjusted according to its blood concentration (maintained at 200–400 ng/mL).
Supportive therapy
Condensed erythrocyte infusion was performed when haemoglobin (Hb) was < 60 g/L, and platelet infusion carried out with either < 10 × 109/L or > 10 × 109/L accompanied by the occurrence of bleeding or symptoms of intracranial haemorrhage. The patients were treated with granulocyte-stimulating factor (GSF) via subcutaneous injection at an absolute neutrophil count (ANC) < 1.5 × 109/L and thrombopoietin (TPO) at a dose of 15,000 U/day until the platelet count was > 20 × 109/L. In addition, symptomatic anti-infective treatments were administered when patients’s course was complicated by infection.
Efficacy evaluation and engraftment detection
Haematopoietic recovery was defined as an ANC ≥ 0.5 × 109/L for 3 days, Hb ≥ 60 g/L for 7 days without red blood cell transfusion and platelet count ≥ 20 × 109/L for 1 week with no platelet transfusion. The CR and PR rates were evaluated after 3, 6 and 12 months of treatment according to the 2009 Guidelines for the Diagnosis and Management of Aplastic Anaemia [11]. Recurrence was defined as a decrease in routine blood indices from the original pretreatment levels of patients with therapeutic responses and efficacy lasting for at least 3 months or the recurrence of blood product dependence. However, recurrence was not deemed if a temporary slight decrease in routine blood indices caused by reducing CsA was observed and the index returned to pre-original treatment levels after using CsA. The patients would be considered nonresponsive if they died without completing 12 months treatment and still incorporating into the efficacy assessment.
Engraftment was confirmed in IIST-UCB group by short tandem repeat polymerase chain reaction (STR-PCR) at the time of granulopoietic recovery and 3 and 6 months after the start of the treatment.
Follow-ups
The primary endpoint was the ORR 1 year after treatment. The secondary endpoints were the recovery time of the neutrophil, platelet counts and Hb, the complication rates, OS and EFS.
Day 0 means the day UCB was infused for IIST-UCB group, while the first day after r-ATG for IST group. A 1-year follow-up was required for all patients in both groups where the treatment turned out to be effective. The day of relapse or death marked the end of the follow-up if SAA or VSAA relapsed or patients died within 1 year. Blood tests and liver and kidney function were performed at least twice a week and monthly respectively before the therapeutic response occurred. Bone marrow cell morphology, cytogenetics and PNH clonal tests of peripheral blood cells via flow cytometry were performed to evaluate the efficacy and to monitor disease progression at 3, 6 and 12 months after treatment. The last follow-up was in December 2018 and median follow-up of 12 (0.5 to 12) months. The OS time refers to the duration from diagnosis to death or the last follow-up. The EFS time refers to the duration from the day of diagnosis to any event or the last follow-up. The events of interest included treatment failure, relapse, new clonal chromosomal abnormalities, PNH, MDS/AML transformation, solid tumours and death.
Statistics
SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) software was used for the statistical analyses. The data was presented as the means ± standard deviations, medians (interquartile ranges) or counts (percentages). Differences between the groups were detected by t-tests, Wilcoxon rank-sum tests, or chi-squared tests. The Kaplan–Meier curve analysis and the log-rank test were performed to compare the haematopoietic recovery times and OS of patients between the two groups. Univariate Cox proportional hazards regression was conducted to assess the factors affecting OS, and logistic regression analysis was performed to evaluate the factors predicting EFS.
Results
Clinical characteristics
The baseline clinical characteristics of the patients are shown in Table 1. There was a significant difference in age between the two groups (P = 0.001), but no significant difference in disease severity, sex or CsA treatment. There were 41 males and 28 females in IIST-UCB group. Among them, 52 patients were diagnosed with SAA, and 17 patients had VSAA. Twenty-four patients took CsA treatment before the treatment, and 45 patients did not take any medications. In IST group, there were 32 and 22 patients diagnosed with SAA and VSAA respectively. Fourteen patients took CsA before the treatment, while 40 patients did not take any medications. The interval from diagnosis to treatment in IIST-UCB group was significantly longer than in IST group (P = 0.036).
Haematopoietic recovery
The Kaplan–Meier curve analysis and log-rank test were used to further compare the recovery times of the three haematopoietic lines between two groups. Significant differences were found in the ANC recovery time between two groups (P = 0.035). The recovery time to Hb ≥ 60 g/L in IIST-UCB group was also significantly shorter than in IST group (P = 0.010). Similarly, the rate of PLT ≥ 20 × 109/L in IIST-UCB group was also significantly higher than that in IST group (P = 0.006), as shown in Fig. 1. Significant differences were also observed in the blood transfusion volume and platelet transfusion volume between two groups. The median blood transfusion volume of IIST-UCB group was 11.0 (6.0–20.0) U, which was significantly less than that of IST group, which was 15.5 (10.0–30.25) U, P = 0.010. In terms of the number of platelets transfused, the median platelet volume for IIST-UCB group was 10.0 (8.0–16.0) therapeutic doses, which was significantly less than IST group, which was 16.5 (11.0–25.5) therapeutic doses, P < 0.001, as shown in Table 2.
ORR, CR and PR
According to the 2009 Guidelines for the Diagnosis and Management of Aplastic Anaemia, the ORR of IIST-UCB group after treatment for 3 months was significantly higher than that of IST group (69.67% vs 51.85%, P = 0.045). In addition, the CR rate was higher in IIST-UCB group than in IST group (21.74% vs 3.7%, P = 0.004). However, there was no significant difference in the PR rate between thetwo groups (47.83% vs 48.15%, P = 0.972), as shown in Table 3.
The ORR was still significantly higher in IIST-UCB group than in IST group (76.81% vs 57.41%, P = 0.022) 6 months after treatment. The CR rate of IIST-UCB group was higher than IST group (37.68% vs 11.11%, P = 0.001). No significant difference was observed in PR rate between two groups (39.13% vs 46.30%, P = 0.425), as shown in Table 3.
Twelve months after treatment, the ORR of IIST-UCB group was still significantly higher than that group (85.51% vs 61.11%, P = 0.002). The CR rate of IIST-UCB group was higher than IST group (59.42% vs 25.93%, P = 0.000). No significant difference was observed in the PR rate between two groups (26.09% vs 35.19%, P = 0.275), as shown in Table 4.
Engraftment
Through STR-PCR, mixed chimerism was detected in 27 patients in IIST-UCB group (39.13%), 2 of which retained 60% of the chimerism after treatment. The other patients who responded to the treatment were ultimately tested as full recipients, indicating self-haematopoietic recovery. In addition, no significant differences in response rates in patients who did or did not have mixed chimerism after we compared those two populations in the IIST-UCB group (Table 5). The median (IQR) of CD34-positive cell count in the IIST-UCB group was 2.25 (1.43–3.21) × 105/kg. The median (IQR) of CD34-positive cell count in the IIST-UCB group with mixed chimerism and without mixed chimerism was 2.46 (1.64–3.09) × 105/kg and 2.21 (1.34–3.32) × 105/kg, respectively. Furthermore, no significant difference was found in the CD34-positive cell count after 3, 6 and 12 months in the IIST-UCB group between patients with mixed chimerism and without mixed chimerism (Table 6).
Safety
The incidence of complications in the two groups was further analysed. No significant differences were observed between the two groups in complications, which included severe infections (P = 0.61), cytomegaloviremia (P = 0.08), Epstein–Barr viremia (P = 0.06) and ATG-related serum diseases (P = 0.77), as shown in Fig. 2. Severe infection referred to infections occurred around the lung, gastrointestinal tract, urinary system and perianal abscess that were not suppressed by anti-infective treatment for > 3 days.
Survival rate analysis and univariate and multivariate analyses of impact factors
Ten of 123 patients died, five in IIST-UCB group and five in IST group, with a median of 90 (12–300) days before death. There was no significant difference in the OS rate between two groups. Regarding EFS rates, the rate was significantly higher in IIST-UCB group than in IST group (P = 0.006), as shown in Fig. 3. The results of univariate and multivariate analyses indicated that severe infection was an independent risk factor for patient survival (P < 0.05) (Table S1). Univariate analysis suggested that treatment with IIST-UCB (P = 0.009), blood transfusion volume (P = 0.000), platelet transfusion volume (P = 0.000) and severe infection (P = 0.000) were important factors affecting the EFS rate. Multivariate analysis suggested that treatment with IIST-UCB (P = 0.037), blood transfusion volume (P = 0.000) and severe infections (P = 0.022) were independent risk factors affecting the EFS rate, as shown in Table S2.
Discussion
Related studies have shown that the mechanism of SAA, especially acquired or idiopathic SAA, is associated with CD34 + stem cell apoptosis caused by abnormalities in the autoimmune system and the attenuation of CD34 + stem cell differentiation to various blood cell lines [12,13,14]. The CD34 + cell count was low in AA who have required no treatment and have not progressed [15]. At present, the treatment of SAA with a high dose of CTX is still controversial. Some studies reported the follow-up of SAA patients taking high-dose CTX for 10 years [16] and showed 10-year overall survival rate was 88% for SAA patients treated with high-dose CTX for the first time, with an overall effective rate of 71%, indicating that high-dose CTX is highly effective for SAA. However, some studies showed that high-dose CTX reduced the risks of recurrence and transition to malignant clonal diseases delayed hemogram recovery and increased mortalities from early infections [17], declining the overall survival rate of SAA patients.
Presently, UCB is mainly used in cord blood transplantation treatment for haematological diseases [18], and few studies have investigated it as a supportive treatment for SAA patients. Xie et al. [10] reported that the overall remission rates at 3 months and 1 year after treatment were 61.1% and 86.2%, respectively, and the 3-year OS was 83.3%, which was much better than patients treated with standard IST. According to a comparison made in our centre between therapeutic treatments with ATG + CTX + UCB and HSCT, no significant differences were observed in the 2-year OS rates [19]. In some studies with small sample sizes, autologous cord blood transplantation combined with immunosuppressive agents was used for the treatment of SAA with better haematologic responses. Nonetheless, studies with larger sample sizes are still needed [20, 21]. A study of 93 SAA patients treated by IST-UCB infusion showed that the complete remission rate and overall effective rate of patients with UCB transfusion were remarkably higher than patients treated with IST 6 months after treatment [22]. Moreover, a quicker therapeutic response in ANC and platelet recovery was observed in UCB + IST group. Multivariate analysis suggested that using UCB as a supportive therapy was an independent prognostic factor for higher CR rates and ORRs. In another study including 68 SAA patients, 35 were treated with standard IST, 33 were treated with standard IST-UCB and both groups achieved the same clinical outcome [23]. All of the above studies indicated that umbilical cord blood played an important role in haematopoietic recovery of SAA patients.
In the above two studies, ATG was administered at a dose of 2.5 mg/kg/day for 5 days. In our study, the therapeutic regimens in IIST-UCB group were ATG 3 mg/kg/day × 5 days and CTX 50 mg/kg/day × 2 days to enhance the immunosuppressive effects. IIST-UCB group displayed a higher ORR and CR rate compared with IST group. IIST-UCB group had faster haematopoietic recoveries, lower blood and platelet transfusion volumes and better therapeutic effects than IST group.
These results suggested that the effects of the therapeutic regimens in IIST-UCB group were much better than those of IST. The reasons could be as follows: (1) ATG combined with CTX in immunosuppressive therapy strengthened the immunosuppressive effects, enabling full immunosuppression [24]; (2) The combination of different immunosuppressive therapies produced synergistic effects and improved the curative effects [25]; (3) UCB is rich in haematopoietic progenitor cells. Therefore, haematopoiesis can be promoted via a temporary implantation of UCB in cases of granulocyte deficiency after UCB transfusion which greatly shortens the time for haematopoietic recovery and improves the bone marrow microenvironment. When granulocyte deficiency overs, CsA can maintain normal haematopoietic function and promote haematopoiesis [26].
In addition, the therapeutic regimen in IIST-UCB group did not increase the occurrence of adverse events based on the analysis of complications and death in this study. The event-free survival rate was significantly higher in IIST-UCB group than in IST group (P = 0.000). A retrospective analysis of over 800 patients treated with IST showed that the 10-year transformation rate of SAA patients to MDS/PNH and other diseases was as high as 10.9% [27]. In that study, follow-up of the two groups’ patients was only carried out for 1 year, and no transformation to clonal diseases was observed. In our previous studies, long-term follow-up was conducted for SAA patients treated with IIST-UCB and no transformation to malignant clonal diseases like MDS and AML was observed.
Severe infection is an important factor in the early death of SAA patients. In this study, nine patients had multiple infections and one died of cyclosporine encephalopathy. The univariate and multivariate analyses suggested that severe infection was an independent risk factor affecting SAA patient survival (P < 0.05). However, there was no significant difference in the incidence of severe infection between the two groups. Although immunosuppression was strengthened by the use of immunosuppressive reagents in IIST-UCB group, there was no increase in the incidence of severe infection in IIST-UCB group which may be due to UCB. A recent study of University of Utah showed that UCB containing a factor named nNIF inhibits the formation of neutrophil extracellular traps (NETs) and is able to treat inflammation and sepsis in vitro and in infected mouse models [28]. A significant difference was observed in the OS rate of mice with sepsis between the group treated with nNIF and the group that was not indicating that UCB played an important role in IST infection. In addition, more attention should be paid to the duration of neutropenia and risk of fungal infections, after infusion high dose of CTX like 100 mg/kg.
In the past decade, with the development of transplantation technologies, alternative donor transplantation has been widely used for SAA treatment [29, 30], especially haploid HSCT [31]. Studies have shown that it has been increasingly used for children and adolescents with SAA and no significant difference in the overall survival rate of SAA patients between treating with allo-HSCT and IST. However, the EFS rate was significantly higher in allo-HSCT group than in IST group [32, 33]. Xu et al. [34] reported that the effects of allo-HSCT were also better than IST for adults. However, compared with HLA-matched HSCT, the incidence of complications like acute and chronic graft-versus-host disease (GVHD) after allo-HSCT was higher, thus adversely affecting the patients’ quality of life [35]. Engraftment detection was performed for patients in IIST-UCB group using STR-PCR in our study. Although two patients had mixed chimerism, neither of them had acute or chronic GVHD and the remaining patients showed haematopoietic self-recovery.
Although the response rate of IIST-UCB is better than that of IST, there remains a possibility that the smaller percentage of VSAA in IIST-UCB group than in IST group (24.64% vs 40.74%) may have affected the response rate. Our results may need to be verified by multicentre trial with large sample size. However, based on all the aforementioned results and the conclusion of our previous research [10], we still suggested to choose IIST-UCB as the first-line treatment compared to IST when there is no HLA-identical donor.
Conclusion
In summary, UCB was shown to be beneficial for haematopoietic recovery, the bone marrow haematopoietic microenvironment and severe infection IST. Moreover, it showed the advantages including a lower requirement for HLA matching, the absence of related complications like GVHD, abundant sources and frequent availability when compared to HSCTUCB. Our research indicated IIST-UCB could serve as a safe and effective therapy for SAA when there is no HLA-identical donor.
Data availability
Not applicable.
Code availability
Not applicable.
References
Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC (2016) British Society for Standards in Haematology Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol 172(2):187–207
Shah S, Jain P, Shah K, Patel K, Parikh S, Patel A, Panchal H, Anand A (2019) Immunosuppressive therapy for aplastic anemia: a single-center experience from western India. Ann Hematol 98(1):41–46
Damodar S (2015) Immunosuppressive therapy for aplastic anaemia. J Assoc Physicians India 63(3 Suppl):16–20
Assi R, Garcia-Manero G, Ravandi F, Borthakur G, Daver NG, Jabbour E, Burger J, Estrov Z, Dinardo CD, Alvarado Y, Hendrickson S, Ferrajoli A, Wierda W, Cortes J, Kantarjian H, Kadia TM (2018) Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer 124(21):4192–4201
Peslak SA, Olson T, Babushok DV (2017) Diagnosis and treatment of aplastic anemia. Curr Treat Options Oncol 18(12):70
Young NS (2018) Aplastic anemia. N Engl J Med 379(17):1643–1656
Ogawa S (2016) Clonal hematopoiesis in acquired aplastic anemia. Blood 128(3):337–347
Babushok DV, Perdigones N, Perin JC, Olson TS, Ye W, Roth JJ, Lind C, Cattier C, Li Y, Hartung H, Paessler ME, Frank DM, Xie HM, Cross S, Cockroft JD, Podsakoff GM, Monos D, Biegel JA, Mason PJ, Bessler M (2015) Emergence of clonal hematopoiesis in the majority of patients with acquired aplastic anemia. Cancer Genet 208(4):115–128
Knudtzon S (1974) In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood 43(3):357–361
Xie LN, Fang Y, Yu Z, Song NX, Kong FS, Liu XM, Zhou F (2014) Increased immunosuppressive treatment combined with unrelated umbilical cord blood infusion in children with severe aplastic anemia. Cell Immunol 289(1–2):150–154
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J, Killick SB, Stewart R, Yin JA (2009) British Committee for Standards in Haematology Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 147(1):43–70
Liu CY, Fu R, Wang HQ, Li LJ, Liu H, Guan J, Wang T, Qi WW, Ruan EB, Qu W, Wang GJ, Liu H, Wu YH, Song J, Xing LM, Shao ZH (2014) Fas/FasL in the immune pathogenesis of severe aplastic anemia. Genet Mol Res 13(2):4083–4088
Sheng W, Liu C, Fu R, Wang H, Qu W, Ruan E, Wang G, Liu H, Wu Y, Song J, Xing L, Guan J, Li L, Liu H, Shao Z (2014) Abnormalities of quantities and functions of linker for activations of T cells in severe aplastic anemia. Eur J Haematol 93(3):214–223
Young NS (2013) Current concepts in the pathophysiology and treatment of aplastic anemia. Hematol Am Soc Hematol Educ Program 2013(1):76–81
Matsui WH, Brodsky RA, Smith BD, Borowitz MJ, Jones RJ (2006) Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia 20(3):458–462
Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, Smith BD, Matsui WH, Goodman SN, Ambinder RF, Jones RJ (2010) High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood 115(11):2136–2141
Zhang F, Zhang L, Jing L, Zhou K, Wang H, Peng G, Li Y, Li Y, Li J, Ye L, Shi L, Fan H, Zhao X, Chu Y, Hao Y, Wang J (2013) High-dose cyclophosphamide compared with antithymocyte globulin for treatment of acquired severe aplastic anemia. Exp Hematol 41(4):328–334
Peffault de Latour R, Chevret S, Jubert C, Sirvent A, Galambrun C, Ruggeri A, Gandemer V, Cornillon J, Rialland F, Dalle JH, Forcade E, Bruno B, Paillard C, Rorlich PS, Salmon A, Fürst S, Sicre de Fontbrune F, Rubio MT, Bay JO, Mohty M, Larghero J, Gluckman E, Socié G (2018) Francophone Society of Bone Marrow Transplantation and Cellular Therapy Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood 132(7):750–754
Yu Z, Zhou F, FuGe L, Liu XM, Fang Y, Xie L, Kong FS, Song NX (2013) High-dose immunosuppressive therapy combined with cord blood infusion and non-myeloablative peripheral blood stem cell transplantation for patients with severe aplastic anemia. Eur Rev Med Pharmacol Sci 17(19):2613–2618
Sun Y, Liu Z, Xiao J, Liu X, Jiang F, Fan S, Shen Y (2019) Autologous cord blood transplantation in children with acquired severe aplastic anemia. Pediatr Transplant 23(1):e13325
Avgerinou G, Oikonomopoulou C, Kaisari A, Ioannidou E, Komitopoulou A, Paisou A, Tourkantoni N, Filippidou M, Kattamis A, Vessalas G, Peristeri I, Goussetis E, Kitra V (2019) Successful long-term hematological and immunological reconstitution by autologous cord blood transplantation combined with post-transplant immunosuppression in two children with severe aplastic anemia. Pediatr Transplant 23(1):e13320
Zhang X, Li Z, Geng W, Song B, Wan C (2018) Effects and predictive factors of immunosuppressive therapy combined with umbilical cord blood infusion in patients with severe aplastic anemia. Yonsei Med J 59(5):643–651
Luo X, Lu H, Xiu B, Wu H, Li B, Li P, Chen Y, Zhou L, Zhang W, Dong Y, Liang A, Ding Y (2018) Efficacy and safety of combined immunosuppressive therapy plus umbilical cord blood infusion in severe aplastic anemia patients: a cohort study. Exp Ther Med 15(2):1966–1974
Schoettler ML, Nathan DG (2018) The pathophysiology of acquired aplastic anemia: current concepts revisited. Hematol Oncol Clin North Am 32(4):581–594
Gupta V, Eapen M, Brazauskas R, Carreras J, Aljurf M, Gale RP, Hale GA, Ilhan O, Passweg JR, Ringdén O, Sabloff M, Schrezenmeier H, Socié G, Marsh JC (2010) Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica 95(12):2119–2125
Yu Z, Zhou F, Ge LF, Liu XM, Fang Y, Xie LN, Kong FS, Song NX, Yu QQ (2015) Mechanism of immunosuppressants combined with cord blood for severe aplastic anemia. Int J Clin Exp Med 8(2):2484–2494
Li Y, Li X, Ge M, Shi J, Qian L, Zheng Y, Wang J (2011) Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol 90(5):529–537
Yost CC, Schwertz H, Cody MJ, Wallace JA, Campbell RA, Vieira-de-Abreu A, Araujo CV, Schubert S, Harris ES, Rowley JW, Rondina MT, Fulcher JM, Koening CL, Weyrich AS, Zimmerman GA (2016) Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J Clin Invest 126(10):3783–3798
Bacigalupo A (2018) Alternative donor transplants for severe aplastic anemia. Hematol Am Soc Hematol Educ Program 2018(1):467–473
Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, Yabe H, Tsuchida M, Mugishima H, Ohara A, Morimoto A, Otsuka Y, Ohga S, Bessho F, Nakahata T, Tsukimoto I, Kojima S (2008) Japan Childhood Aplastic Anemia Study Group. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood 111(3):1054–1059
Xu LP, Zhang XH, Wang FR, Mo XD, Han TT, Han W, Chen YH, Zhang YY, Wang JZ, Yan CH, Sun YQ, Zuo SN, Huang XJ (2017) Haploidentical transplantation for pediatric patients with acquired severe aplastic anemia. Bone Marrow Transplant 52(3):381–387
Yang S, Yuan X, Ma R, Jiang L, Guo J, Zang Y, Shi J, Yang J, Lei P, Liu Z, Zhang Y, Zhu Z (2019) Comparison of outcomes of frontline immunosuppressive therapy and frontline haploidentical hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched sibling donor. Biol Blood Marrow Transplan 25(5):975–980
Kim H, Im HJ, Koh KN, Kang SH, Yoo JW, Choi ES, Cho YU, Jang S, Park CJ, Seo JJ (2019) Comparable outcome with a faster engraftment of optimized haploidentical hematopoietic stem cell transplantation compared with transplantations from other donor types in pediatric acquired aplastic anemia. Biol Blood Marrow Transplant 25(5):965–974
Xu ZL, Zhou M, Jia JS, Mo WJ, Zhang XH, Zhang YP, Wang Y, Li YM, Huang XJ, Wang SQ, Xu LP (2019) Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia. Bone Marrow Transplan 54(8):1319–1326
Xue W, Jiang M, Tian M, Duan X, Qu J, Yuan H, Xu J, Wen B, Li L, Wang Y, Liu Y, Wang X, Cao H (2014) Clinical features and risk factors analysis of acute graft-versus-host disease in patients with related HLA-haploidentical non T cell-depleted in vitro peripheral hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 35(12):1100–1106
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author information
Authors and Affiliations
Contributions
Fang Zhou finished study design; Fang Zhou, Fengkui Zhang, Li Zhang, Junjie Ma, Chunting Zhao, Ling Wang finished experimental studies; Qian Wu, Guitao Jie, Haiyan Zhang, Hao Zhang, Shunqing Wang, Qingliang Teng finished data analysis; Fang Zhou, Qian Wu finished manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Ethics Committee of the 960 Hospital of Chinese People’s Liberation Army, and all patients signed informed consent forms. The study was conducted in accordance with the Declaration of Helsinki and has been registered on clinicaltrials.gov (No: NCT02838992).
Consent for publication
We had obtained from the patient for written informed consent and any accompanying images for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, F., Zhang, F., Zhang, L. et al. A multicentre trial of intensive immunosuppressive therapy combined with umbilical cord blood for the treatment of severe aplastic anaemia. Ann Hematol 101, 1785–1794 (2022). https://doi.org/10.1007/s00277-022-04864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04864-1