Abstract
The incidence and outcomes of aplastic anemia (AA) in Asia remain limited. This study aimed to explore the incidence and outcomes of patients with adult AA across the country of Thailand. This is a prospective multi-center nationwide population-based observational study of AA patients aged at least 15 years old, diagnosed from August 2014 to July 2016, with a longitudinal follow-up period over 2 years. There were 348 newly diagnosed adult AA patients during the enrollment period, giving an annual incidence of 4.6 per million. The incidence of severe (SAA) and very severe aplastic anemia (VSAA) (3.8 per million) was higher than non-severe AA (NSAA, 0.8 per million). The peak incidence was observed in the patients aged from 80 to 89 years old (14.4 per million). The 2-year overall survival (OS) in NSAA, SAA, and VSAA were 65.5%, 49.3%, and 20.1%, respectively (P < 0.001). With regard to the response to immunosuppressive therapy, the overall response rate (ORR) in SAA/VSAA treated with rabbit anti-thymocyte globulin with/without cyclosporin A (rATG ± CsA) were significantly superior to those treated with CsA alone, or anabolic steroids (44.4% vs 36.4% and 31.2%, respectively, P < 0.001). The 2-year OS in SAA/VSAA treated with rATG ± CsA, CsA, and anabolic steroids were 54.8%, 54.5%, and 37.6% (P = 0.037), respectively. The incidence of adult AA in Thailand is higher than those in Western countries, and the peak incidence is in the elderly. rATG ± CsA provided a better response than anabolic steroids, translating to the superior survival in SAA/VSAA treated with rATG ± CsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of aplastic anemia (AA) varied worldwide. The estimated annual incidence in Western countries was 1.5–2.3 per million [1,2,3,4,5], while it was 2–3 times higher in Asia (3.0–7.5 per million) [6,7,8,9]. The explanation for these differences is unknown; however, it may be related to genetic or environmental factors. The relationship between AA and environmental exposure including certain medications, benzene, contaminated water sources, animal fertilizers, and pesticides was previously reported [10, 11].
Immunosuppressive therapy (IST) is recommended for the treatment of SAA patients who are not eligible for hematopoietic stem cell transplantation (HSCT) [12, 13]. Although horse ATG (hATG) gives better responses over rabbit ATG (rATG) as first-line therapy [13], rATG is widely used as initial therapy in Europe and Asia due to unavailability of the hATG. Recent multi-national studies of rATG in Europe and Asia showed 1-year response rates of 63–65% with favorable survival outcomes (2-year overall survival 79.0–86.3%) [14,15,16]. Anabolic steroids were used as an alternative therapy for those unaffordable or unsuitable for HSCT or IST. To understand the current situation of adult AA in Thailand, the annual incidence of AA across the country and the treatment responses as well as survival outcome according to patient’s characteristics and treatment modalities were studied.
Methods
Study design and patients
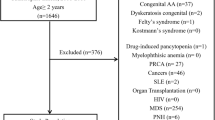
This study was a multi-center prospective observational study. Newly diagnosed adult AA patients from 30 referral centers nationwide (aged at least 15 years) were enrolled during the period from August 1, 2014, to July 31, 2016. Criteria for diagnosis of AA included bone marrow hypocellularity with at least two of the following: anemia with corrected reticulocyte count ≤ 1%, absolute neutrophil count (ANC) ≤ 1.5 × 109/L, or platelet count ≤ 50 × 109/L. Patients with myelodysplastic syndrome (MDS) or classic paroxysmal nocturnal hemoglobinuria and chemotherapy-induced bone marrow suppression were excluded. Patients with chromosomal abnormalities were also excluded. Those with nationalities other than Thai were excluded. All enrolled patients were reassessed for definite diagnosis by the central review committee. Treatment of choice was given at the discretion of the treating physicians, depending on the patient’s eligibility and accessibility to treatment. The research proposal and all subsequent amendments were approved by the local Ethics Committee at each study site. The study was conducted in accordance with ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All patients provided written informed consent.
Definition
SAA has at least 2 of the following: reticulocyte count ≤ 1%, ANC ≤ 0.5 × 109/L, platelet count ≤ 20 × 109/L, and hypocellular bone marrow with ≤ 25% cellularity. VSAA fulfills the definition of SAA but with ANC ≤ 0.2 × 109/L. Those who do not meet the criteria for SAA/VSAA are NSAA.
Responses
Responses to treatment were evaluated at each visit every 3 months. In those who switched to subsequent therapy, the responses were reassessed to evaluate the efficacy of the second-line treatment. Criteria for responses were based on the British Committee for Standard Haematology (BCSH) guideline [12, 17]. Briefly, the response criteria were as follows: complete response (CR): normal hemoglobin (Hb) for age and gender with ANC ≥ 1.5 × 109/L and platelet ≥ 150 × 109/L; partial response (PR): transfusion independence and no longer met the criteria for severe disease, and no response (NR): not achieving CR/PR.
The response criteria for NSAA were similar to SAA for CR and NR, whereas the criteria for PR were transfusion independence (if previously dependent) or doubling or normalization of at least one lineage or increase of baseline Hb ≥ 3 g/dL, ANC ≥ 0.5 × 109/L (if initially < 0.5 × 109/L), and platelet ≥ 20 × 109/L (if initially < 20 × 109/L).
Statistical analyses
The incidence rate was calculated by the number of newly diagnosed AA patients divided by the number of inhabitants within the respective catchment areas. All eligible cases were recruited from almost all hospitals over the country. We excluded hospitals in which there were no hematologists. The catchment areas were defined to only the provinces with the hematology services (Fig. 1). Only patients who had lived at least 6 months in the catchment area were included into the incidence calculation. Population numbers were collected from the national database provided by the Strategy and Planning Division, Office of Permanent Secretary, Ministry of Public Health (http://bps.moph.go.th/new_bps/). Direct age standardization was presented as previously described [18].
Comparisons of data were performed using t-test or Wilcoxon rank-sum test or χ2, depending on variable types and data distribution. OS was analyzed using Kaplan–Meier plots. Cox regressions were used to estimate hazard ratios among given subgroup stratifications. Treatment responses to immunosuppressive therapy were pre-defined as previously described criteria [17]. The statistical analyses were performed using SAS 9.4.
Results
A total of 348 patients were enrolled. Twenty-six patients were excluded for the incidence calculation because they had lived in the catchment area less than 6 months. Six patients were excluded from the treatment response analyses due to inadequate collected data.
Incidence of AA
There were 322 patients with newly diagnosed adult AA during the 2-year periods, which yielded an overall annual incidence of 4.6 per million. The annual incidence of SAA/VSAA was 3.8 per million, higher than that of NSAA (0.8 per million with a ratio of 4.6:1). Forty-five patients had VSAA, giving the annual incidence of 0.6 per million. The incidence was high in older patients with the peak of 14.4 per million in patients aged from 80 to 89 years old (Table 1). It was lower in patients aged less than 50 (1.2–2.3 per million). There was a slightly higher incidence in men than women (4.8 vs 4.0 per million). Age-standardized incidence rates (ASRs) were reported by geographical regions, which showed the highest incidences in eastern and north-eastern areas with ASRs of 6.6 and 5.6 per million, respectively. Variation in incidence rate was observed across the country, ranging from 2.6 to 6.6 per million (Fig. 1, Table S1).
Patients’ characteristics
With regard to the severity, there were 45 patients (13.1%), 238 (69.5%), and 59 (17.2%) with VSAA, SAA, and NSAA, respectively (Table S2). The majority of the patients (82.6%) were considered at least severe. The median age was 59 years old (range, 15–93). Seventy-two percent (72%) of them aged at least 50 years. Patients with VSAA had more severe manifestation: i.e., infections (44.4% in VSAA vs 16.8% in SAA vs 11.9% in NSAA, P = 0.005) and bleeding (80.0% in VSAA vs 59.2% in SAA vs 40.7% in NSAA, P = 0.0003). Frequent environmental exposures were agriculture pesticides (n = 60), certain medications previously reported as possible cause of AA within 6 months (n = 32), ducks/geese, and animal fertilizer (n = 15).
Treatment modalities
Of 283 SAA/VSAA patients, only 3 patients underwent HSCT, 143 received rATG ± CsA, 12 CsA only, 102 anabolic steroids as the frontline therapy, and supportive treatment in 23 patients (Table 2). For rATG treatment, it was frontline treatment in 143 patients and additionally as second line in 10 patients. Twenty patients underwent second rATG treatment after failure or relapse to the first rATG.
One hundred thirty-seven (137) patients with SAA/VSAA did not receive frontline rATG. The reasons included patient conditions not suitable for IST (such as active infections and comorbidities, n = 58), inaccessibility to rATG (n = 35), concerns regarding medical facility (n = 17), and other reasons (n = 27).
In 59 NSAA patients, the majority of them (n = 49, 83.1%) received anabolic steroids as frontline therapy, while 8 received supportive treatment. The other 2 patients received CsA.
Treatment response in patients with SAA/VSAA
Two-hundred eighty patients were evaluated for their treatment responses. Patients with VSAA had inferior ORR than those with SAA (15.6% vs 40.0%, P = 0.002) (Table S4A). The ORR among patients treated with rATG ± CsA (44.4%) was significantly superior than those treated with CsA-based treatment (36.4%) and anabolic steroids (31.2%) (P < 0.001). The ORR of first treatment with rATG ± CsA (n = 153) at 3, 6, 12, and 24 months were 17.6% (95% CI, 12.0–24.6), 30.1% (95% CI, 22.9–38.0), 34.0% (95% CI, 26.5–42.1), and 37.9% (95% CI, 32.0–46.1), respectively (Table 3). The ORR among evaluable patients were 23.9%, 43.8%, 68.4%, and 89.2% at 3, 6, 12, and 24 months, respectively. Among 20 patients who received second rATG ± CsA, 1-year ORR was 25% (95% CI, 6.0–44.0). From multivariate analysis, anabolic steroids had lower ORR than rATG ± CsA with an odds ratio (OR) of 0.57 (95% CI, 0.33–0.98, P = 0.04) (Table S4A).
The ORR to rATG ± CsA was lower in patients older than 60 years (32.0% vs 50.5% for ≤ 60 years, P = 0.02) while patients with VSAA had a trend of a poorer response than those with SAA (23.5% vs 47.1%, P = 0.06) (Table S5). At 24 months, the rATG dose range of 3.0–3.5 mg/kg/day (39.7%) and 3.5–3.75 mg/kg/day (46.9%) provided a better response rate than that of the lower dose range of 2.5–3.0 mg/kg/day (21.4%, P = 0.03) (Fig. 2). Nevertheless, the serious uncommon adverse events of the higher dose range of 3.5–3.75 mg/kg/day were observed including hepatotoxicity (n = 2), acute renal failure (n = 2), and cardiac arrest (n = 1) (Table S6).
Overall response rate to rATG ± CsA in SAA/VSAA at each time point according to rATG dosage. Y-axis represented the overall response rate. Z-axis represented time point of response evaluation after treatment with rATG. X-axis represented rATG dosage range (mg/kg/day). * denotes statistical significance with P-value of 0.038
Treatment response in patients with NSAA
Of 49 patients with NSAA who were treated with anabolic steroids, 20 patients (40.8%) responded to treatment with a CR rate of 6.1% (n = 3). The ORR of anabolic steroids at 3, 6, 12, and 24 months were 28.6% (95% CI, 16.6–43.3%), 34.7% (95% CI, 21.7–49.6%), 40.8% (95% CI, 27.0–55.8%), and 34.7% (95% CI, 21.7–49.6%), respectively (Table S3).
Survival outcome of all patients
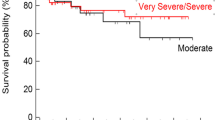
Among 342 patients with AA, the median OS was 19.2 months (95% CI, 12.9 months-not reached) with a 2-year OS of 48.1% (95% CI, 45.3–50.4). When stratified by the disease severity, the 2-year OS for NSAA, SAA, and VSAA were 65.5% (95% CI, 59.0–72.0), 49.3% (95% CI, 46.0–52.6), and 20.1% (95% CI, 14.0–26.2), respectively (P < 0.001) (Fig. 3A). The 2-year OS for patients aged 15–40, 41–60, and > 60 years were 64.5% (95% CI, 58.0–71.0), 47.7% (95% CI, 43.1–49.3), and 42.6% (95% CI, 38.6–46.6) (P = 0.02), respectively (Fig. 3B). There was no significant difference in survivals between gender with 2-year OS in males and female of 50.4% (95% CI, 46.5–54.3) and 45.7% (95% CI, 41.7–49.7), respectively (P = 0.24) (Fig. 3C).
Survival outcome of patients with SAA/VSAA
The 2-year OS of patients treated with rATG ± CsA, CsA-based treatment, anabolic steroids, and non-specific therapy were 54.8% (95% CI, 50.7–58.9), 54.5% (95% CI, 39.5–69.5), 37.6% (95% CI, 32.5–42.7), and 0% (P < 0.001), respectively (Fig. 4A). rATG ± CsA provided a superior OS over anabolic steroids (hazard ratio; HR 1.56, 95% CI, 1.10–2.23, P = 0.013) and non-specific treatment (HR 6.83, 95% CI, 4.11–11.36, P < 0.001). From multivariate analysis, age > 60 years (HR 1.63, 95% CI, 1.14–2.33, P = 0.007), VSAA (HR 2.24, 95% CI, 1.45–3.46, P < 0.001), and non-specific treatment (HR 4.96, 95% CI, 2.88–8.54, P < 0.001) were independently associated with inferior OS among patients with SAA/VSAA (Table S4B). Notably, patients who attained response after rATG ± CsA had a superior OS than non-responder with HR of 47.5 (95% CI, 11.6–195.3, P < 0.001) (Fig. 4B).
Discussion
In this nationwide prospective study of adult AA patients diagnosed from 2014 to 2016, the annual incidence of adult AA in Thailand was 4.6 per million, which considerably is more or less similar to 3.0–5.0 per million in the previous population-based study in Thailand conducted 20 years ago [6]. Similarity in the annual incidence is also noted among regional parts: 2.8 per million in the southern region (vs 3.0 per million in Songkla and nearby provinces) and 5.6 per million in the north-eastern region (vs 5.0 per million in Khon Kaen and nearby provinces) [6]. The steadiness of the incidence through decades, even the changes in lifestyle and environment toward urbanization, favors the genetic predisposition of autoimmunity as the disease etiology [19, 20]. Nevertheless, the environmental exposer may have an impact on the variation of geographical incidence as the lowest was in the central region (2.5 per million), whereas the incidence was higher in the north-eastern region, the largest agricultural area (5.6 per million) and the peak was in the eastern region, the major industrial district (6.5 per million). The AA annual incidence of 4.6 per million being reported here is approximately twice higher than that from Western countries (ranged 1.5–2.3) [1,2,3,4,5] but is similar to the reports from Asia: 4.8 in Malaysia [21], 5.7 in Taiwan [9], and 7.5 in China [8, 22].
The current study demonstrated a remarkable age-related distribution with steeply increased incidence after the age of 50 (6.2 per million) with a peak incidence at the age over 80 years old (14.3 per million). The pre-existing clonal hematopoiesis, causing bone marrow failure with overlapping features of hypocellular MDS and AA, may lead to a higher incidence of AA among elderly patients [23, 24]. Due to a high proportion of patients with SAA/VSAA in this study (83%) and half of them older than 60 years, the 2-year OS was only 48%, compared with the other reports from Sweden (5-year OS 90.7%) [5], Taiwan (5-year OS 60%) [9], and Spain (2-year OS 57%) [3]. Following the previous population-based studies [3, 5, 9], older age and severity of the disease were the strong predictors of inferior survival among patients with AA.
HSCT is recommended treatment for younger patients of less than 40–50 years old. However, not all eligible patients could undergo this expensive procedure. Furthermore, the government supports only specific number of patients. Therefore, only small number of patients underwent HSCT in this cohort.
rATG is the mainstay of treatment for SAA in Thailand as well as in many countries in Asia. For patients with SAA/VSAA in this cohort, the ORR was considerably low (44.4%), compared with previously published reports of rATG [14, 16]. However, when considering the response among the evaluable patients, the response gradually increased over time with the ORR of 44% at 6 months and 68% at 1 year, consistent with the reports of rATG from Asia (ORR 17% at 6 months and 64% at 12 months) and European Blood and Marrow Transplant (EBMT) (ORR 52% at 6 months and 64% at12 months) [14, 16]. Although the response to rATG in this study was delayed, it was increased over time and the favorable response of rATG was a surrogate marker of long-term survival, similar to previous reports [14, 25].
Anabolic steroids have long been used to treat AA. Its mechanisms include increasing telomerase (TERT) gene expression in hematopoietic cells [26], stimulating erythroid progenitor [27], and enhancing regulatory T-cell [28]. The responses to anabolic steroids are varied, ranging from 25 to 56%, depending on the severity of AA and the treatment combination [29,30,31,32]. In this cohort, the response to anabolic steroids appeared to improve over time with an acceptable response in patients with NSAA (40.8%). In SAA/VSAA, the responses were unfavorable (31.2%) compared with rATG ± CsA (44.4%). Giving the inferior OS among patients treated with anabolic steroids, anabolic steroids should therefore be reserved for patients with NSAA and those with SAA/VSAA who are unfit for IST and HSCT.
The outcomes of this study represented the real-world situation of patients with AA. The cases were collected from 30 hospitals, including university and public hospitals, located in all regions of the country; therefore, the data would reasonably reflect the actual AA incidence in Thailand. The further strengths of this study included the prospective design and centrally reviewed diagnosis. However, this study had some limitations. First, the incidence of NSAA may be underestimated especially non-transfusion dependent AA and asymptomatic cases. Second, there was a proportion of non-evaluable patients due to death and loss to follow-up. Third, this study was not randomized, and the comparison between treatment modalities should therefore be interpreted with caution as there would be a high selection bias for older and unfit patients to receive anabolic steroids or supportive care.
In conclusion, the incidence of adult AA in Thailand from this nationwide population-based study is higher than in Western countries, and even higher among the elderly. The real-world outcome of patients with SAA, especially in those aged over 60 years, is substantially poor. The appropriate therapeutic options, as well as the accessibility to advanced treatment, are needed to ensure a better outcome of patients.
Data availability
Yes.
Code availability
Not applicable.
References
Maluf E, Hamerschlak N, Cavalcanti AB, Junior AA, Eluf-Neto J, Falcao RP, Lorand-Metze IG, Goldenberg D, Santana CL, Rodrigues Dde O, Passos LN, Rosenfeld LG, Pitta M, Loggetto S, Ribeiro AA, Velloso ED, Kondo AT, Coelho EO, Pintao MC, de Souza HM, Borbolla JR, Pasquini R (2009) Incidence and risk factors of aplastic anemia in Latin American countries: the LATIN case-control study. Haematologica 94(9):1220–1226. https://doi.org/10.3324/haematol.2008.002642
Mary JY, Baumelou E, Guiguet M (1990) Epidemiology of aplastic anemia in France: a prospective multicentric study. The French Cooperative Group for Epidemiological Study of Aplastic Anemia. Blood 75(8):1646–1653
Montane E, Ibanez L, Vidal X, Ballarin E, Puig R, Garcia N, Laporte JR (2008) Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica 93(4):518–523. https://doi.org/10.3324/haematol.12020
Tweddle D, Reid M (1996) Aplastic anaemia in the northern region of England. Acta Paediatr 85(11):1388–1389. https://doi.org/10.1111/j.1651-2227.1996.tb13938.x
Vaht K, Goransson M, Carlson K, Isaksson C, Lenhoff S, Sandstedt A, Uggla B, Winiarski J, Ljungman P, Brune M, Andersson PO (2017) Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica 102(10):1683–1690. https://doi.org/10.3324/haematol.2017.169862
Issaragrisil S (1999) Epidemiology of aplastic anemia in Thailand. Thai Aplastic Anemia Study Group. Int J Hematol 70(3):137–140
Kojima S (2002) Aplastic anemia in the Orient. Int J Hematol 76(Suppl 2):173–174. https://doi.org/10.1007/bf03165112
Yang C, Zhang X (1991) Incidence survey of aplastic anemia in China. Chin Med Sci J 6(4):203–207
Li SS, Hsu YT, Chang C, Lee SC, Yen CC, Cheng CN, Chen JS, Lin SH, Chang KC, Chen TY (2019) Incidence and treatment outcome of aplastic anemia in Taiwan-real-world data from single-institute experience and a nationwide population-based database. Ann Hematol 98(1):29–39. https://doi.org/10.1007/s00277-018-3486-3
Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Leaverton PE, Shapiro S, Young NS (2006) The epidemiology of aplastic anemia in Thailand. Blood 107(4):1299–1307. https://doi.org/10.1182/blood-2005-01-0161
Young NS, Kaufman DW (2008) The epidemiology of acquired aplastic anemia. Haematologica 93(4):489–492. https://doi.org/10.3324/haematol.12855
Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC, British Society for Standards in H (2016) Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol 172(2):187–207. https://doi.org/10.1111/bjh.13853
Pulsipher MA, Young NS, Tolar J, Risitano AM, Deeg HJ, Anderlini P, Calado R, Kojima S, Eapen M, Harris R, Scheinberg P, Savage S, Maciejewski JP, Tiu RV, DiFronzo N, Horowitz MM, Antin JH (2011) Optimization of therapy for severe aplastic anemia based on clinical, biologic, and treatment response parameters: conclusions of an international working group on severe aplastic anemia convened by the Blood and Marrow Transplant Clinical Trials Network, March 2010. Biol Blood Marrow Transplant 17(3):291–299. https://doi.org/10.1016/j.bbmt.2010.10.028
Chuncharunee S, Wong R, Rojnuckarin P, Chang CS, Chang KM, Lu MY, Hwang WL, Koh LP, Chen TY, Leung AY, Norasetthada L, Wang SC, Chang MC, Wu KH, Issaragrisil S (2016) Efficacy of rabbit antithymocyte globulin as first-line treatment of severe aplastic anemia: an Asian multicenter retrospective study. Int J Hematol 104(4):454–461. https://doi.org/10.1007/s12185-016-2053-8
Sasaki N, Shimura K, Yoshida M, Uoshima N, Kiyota M, Hatsuse M, Uchiyama H, Chinen Y, Kobayashi T, Nakao M, Takahashi R, Nakano-Akamatsu S, Kaneko H, Kobayashi Y, Shimazaki C, Taniwaki M, Kuroda J, Kyoto Clinical Hematology Study Group i (2019) Immunosuppressive therapy with rabbit antithymocyte globulin therapy for acquired aplastic anemia: a multi-institutional retrospective study in Japanese adult patients. Int J Hematol 109(3):278–285. https://doi.org/10.1007/s12185-018-02583-w
Bacigalupo A, Oneto R, Schrezenmeier H, Hochsmann B, Dufour C, Kojima S, Zhu X, Chen X, Issaragrisil S, Chuncharunee S, Jeong DC, Giammarco S, Van Lint MT, Zheng Y, Vallejo C (2018) First line treatment of aplastic anemia with thymoglobuline in Europe and Asia: outcome of 955 patients treated 2001–2012. Am J Hematol 93(5):643–648. https://doi.org/10.1002/ajh.25081
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J, Killick SB, Stewart R, Yin JA (2009) Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 147(1):43–70. https://doi.org/10.1111/j.1365-2141.2009.07842.x
Naing NN (2000) Easy way to learn standardization: direct and indirect methods. Malays J Med Sci 7(1):10–15
Medinger M, Drexler B, Lengerke C, Passweg J (2018) Pathogenesis of acquired aplastic anemia and the role of the bone marrow microenvironment. Front Oncol 8:587–587. https://doi.org/10.3389/fonc.2018.00587
Schoettler ML, Nathan DG (2018) The pathophysiology of acquired aplastic anemia: current concepts revisited. Hematol Oncol Clin North Am 32(4):581–594. https://doi.org/10.1016/j.hoc.2018.03.001
Yong AS, Goh AS, Rahman M, Menon J, Purushothaman V (1998) Epidemiology of aplastic anaemia in the state of Sabah. Malaysia Med J Malaysia 53(1):59–62
Zhu XF, He HL, Wang SQ, Tang JY, Han B, Zhang DH, Wu LQ, Wu DP, Li W, Xia LH, Zhu HL, Liu F, Shi HX, Zhang X, Zhou F, Hu JD, Fang JP, Chen XQ, Ye TZ, Liang YM, Jin J, Zhang FK (2019) Current treatment patterns of aplastic anemia in China: a prospective cohort registry study. Acta Haematol 142(3):162–170. https://doi.org/10.1159/000499065
Ogawa S (2016) Clonal hematopoiesis in acquired aplastic anemia. Blood 128(3):337–347. https://doi.org/10.1182/blood-2016-01-636381
Stanley N, Olson TS, Babushok DV (2017) Recent advances in understanding clonal haematopoiesis in aplastic anaemia. Br J Haematol 177(4):509–525. https://doi.org/10.1111/bjh.14510
Atta EH, Dias DS, Marra VL, de Azevedo AM (2010) Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol 89(9):851–859. https://doi.org/10.1007/s00277-010-0944-y
Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS (2009) Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114(11):2236–2243. https://doi.org/10.1182/blood-2008-09-178871
Shahani S, Braga-Basaria M, Maggio M, Basaria S (2009) Androgens and erythropoiesis: past and present. J Endocrinol Invest 32(8):704–716. https://doi.org/10.1007/BF03345745
Khurana H, Malhotra P, Sachdeva MU, Varma N, Bose P, Yanamandra U, Varma S, Khadwal A, Lad D, Prakash G (2018) Danazol increases T regulatory cells in patients with aplastic anemia. Hematology 23(8):496–500. https://doi.org/10.1080/10245332.2018.1435045
Chuhjo T, Yamazaki H, Omine M, Nakao S (2008) Danazol therapy for aplastic anemia refractory to immunosuppressive therapy. Am J Hematol 83(5):387–389. https://doi.org/10.1002/ajh.21118
Allen DM, Fine MH, Necheles TF, Dameshek W (1968) Oxymetholone therapy in aplastic anemia. Blood 32(1):83–89. https://doi.org/10.1182/blood.V32.1.83.83
Bacigalupo A, Chaple M, Hows J, Van Lint MT, McCann S, Milligan D, Chessells J, Goldstone AH, Ottolander J, van’t Veer ET, Comotti B, Coser P, Broccia G, Bosi A, Locasciulli A, Catalano L, Battista R, Arcese W, Carotenuto M, Marmont AM, Smith ECG (1993) Treatment of aplastic anaemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol 83(1):145–151. https://doi.org/10.1111/j.1365-2141.1993.tb04645.x
Wang W, Wang X, Xu X, Lin G (2011) Diagnosis and treatment of acquired aplastic anaemia in adults: 142 cases from a multicentre, prospective cohort study in Shanghai, China. J Int Med Res 39(5):1994–2005. https://doi.org/10.1177/147323001103900546
Acknowledgements
The authors acknowledge all Thai AA Registry investigators who are not mentioned in the author list. Their names and affiliates are as follows: Busakorn Saeaeng, Banphaeo Hospital; Arunrat Pirunsarn, Bhuddasothorn Hospital; Harutaya Kasyanan, Piyanuch Chantawong, Buddhachinaraj Hospital; Naiyana Panoi, Chonburi Hospital; Rachanid Pornvipavee, Dusit Jitueakul, Faculty of Medicine Vajira Hospital; Komsai Suwanno, Ngamta Saekoo, Hatyai Hospital; Nisa Makruasi, Kaewta Tongdonup, HRH Princess Maha Chakri Sirindhorn Medical Center; Sumonphan Patthanothai, Thanakrit Somprasertkul, Aurawan Doungmungkorn, Khon Kaen Hospital; Chantiya Chanswangphuwana, Suphan Gunsue, Nittaya Boonnak, Supaporn Lertkawinanan, King Chulalongkorn Memorial Hospital; Bunchoo Suntorn-opas, Klang Hospital; Piriyaporn Iamsai, Lampang Hospital; Adisak Tantiworawit, Ekarat Rattarittamrong, Sasinee Hantrakool, Sudarat Mueangcharoen, Maharaj Nakorn Chiang Mai Hospital; Watcharin Yingsitsiri, Kanitta Sae-ung, Maharat Nakhon Ratchasima Hospital; Tidaluck Wongsunsern, Nakhon Phanom Hospital; Peerapon Wong, Akamon Tapprom, Rawisut Deoisares, Naresuan University Hospital; Kannika Kosila, Nong Khai Hospital; Naritsara Wongtong, Panyananthaphikkhu Chonprathan Medical Center; Tontanai Numbenjapon, Chanchai Traivaree, Thanvalai Riabroi, Phramongkutklao Hospital; Mariam Chetanachan, Silchai Phuangsombat, Nutsara Nakpan, King Prajadhipok Memorial Hospital; Tawatchai Suwanban, Rajavithi Hospital; Pimjai Niparuck, Siriwan Anantato, Ramathibodi Hospital; Leelawan Wiboonmongkol, Ratchaburi Hospital; Sukit Banchongkit, Waree Chanthawibun, Saysuda Poka, Rayong Hospital; Prakong Naka-In, Sakonnakhon Hospital; Jane Jianthanakanon, Chutima Kunacheewa, Eakkapol Utchariyaprasit, Sujantra Ittikusumarn, Siriraj Hospital; Pongtep Viboonjuntra, Songklanagarind Hospital; Kanchana Chansung, Chinadol Wanitpongpun, Kamolwan Thepsuthammarat, Srinagarind Hospital; Anoree Surawong, Yuwadee Kitprasong, Sunpasitthiprasong Hospital; Tananchai Akrawikrai, Surin Hospital; Nonglak Kanitsap, Piyanuch Kongtim, Thammasat University Hospital; Supannee Sudsa, Sudawadee Teocharoen, Supaporn Komsrang, Udon Thani Hospital. We acknowledge Atiphu Charoenpiriyanont, Nuengruetai Jaiwongphen, Jaruek Charoensap, and Jirapha Laohawattanakun, Sanofi employees, who provided administrative supports and assistance in manuscript development. Statistical analyses were conducted by Sarahath Lawpoolsri and Pattarakun Pramnoi, BIOPHICS, Faculty of Tropical Medicine, Mahidol University.
Funding
This study was supported by Sanofi-Aventis (Thailand), Ltd. and designed by the sponsor in close collaboration with the Study Steering Committee. The sponsor conducted the statistical analysis. The authors had full access to the data and participated actively in interpreting data and critically reviewing the article with the assistance of the sponsor. All authors approved the final manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by BIOPHICS, Faculty of Tropical Medicine, Mahidol University. The first draft of the manuscript was written by Lalita Norasetthada and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The research proposal and all subsequent amendments were approved by the local Ethics Committees at each study site. The study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
All patients individually provided written informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Norasetthada, L., Wongkhantee, S., Chaipokam, J. et al. Adult aplastic anemia in Thailand: incidence and treatment outcome from a prospective nationwide population-based study. Ann Hematol 100, 2443–2452 (2021). https://doi.org/10.1007/s00277-021-04566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04566-0