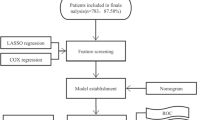
Abstract
Extranodal involvement is a well-known prognostic factor in patients with diffuse large B-cell lymphomas (DLBCL). Nevertheless, the prognostic impact of the extranodal scoring system included in the conventional international prognostic index (IPI) has been questioned in an era where rituximab treatment has become widespread. We investigated the prognostic impacts of individual sites of extranodal involvement in 761 patients with DLBCL who received rituximab-based chemoimmunotherapy. Subsequently, we established a new extranodal scoring system based on extranodal sites, showing significant prognostic correlation, and compared this system with conventional scoring systems, such as the IPI and the National Comprehensive Cancer Network-IPI (NCCN-IPI). An internal validation procedure, using bootstrapped samples, was also performed for both univariate and multivariate models. Using multivariate analysis with a backward variable selection, we found nine extranodal sites (the liver, lung, spleen, central nervous system, bone marrow, kidney, skin, adrenal glands, and peritoneum) that remained significant for use in the final model. Our newly established extranodal scoring system, based on these sites, was better correlated with patient survival than standard scoring systems, such as the IPI and the NCCN-IPI. Internal validation by bootstrapping demonstrated an improvement in model performance of our modified extranodal scoring system. Our new extranodal scoring system, based on the prognostically relevant sites, may improve the performance of conventional prognostic models of DLBCL in the rituximab era and warrants further external validation using large study populations.




Similar content being viewed by others
References
A predictive model for aggressive non-Hodgkin’s lymphoma (1993) The international Non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med 329:987–994
Hui D, Proctor B, Donaldson J et al (2010) Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma 51:1658–1667. doi:10.3109/10428194.2010.504872
Zhou Z, Sehn LH, Rademaker AW et al (2014) An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123:837–842. doi:10.1182/blood-2013-09-524108
Lu CS, Chen JH, Huang TC et al (2015) Diffuse large B-cell lymphoma: sites of extranodal involvement are a stronger prognostic indicator than number of extranodal sites in the rituximab era. Leuk Lymphoma 56:2047–2055. doi:10.3109/10428194.2014.982636
Takahashi H, Tomita N, Yokoyama M et al (2012) Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer 118:4166–4172. doi:10.1002/cncr.27381
Hwang HS, Yoon DH, Suh C, Huh J (2015) Body mass index as a prognostic factor in Asian patients treated with chemoimmunotherapy for diffuse large B cell lymphoma, not otherwise specified. Ann Hematol 94:1655–1665. doi:10.1007/s00277-015-2438-4
Stein H, Chan JKC, Warnke RA et al (2008) Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 233–237
Lister TA, Crowther D, Sutcliffe SB et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7:1630–1636
Armitage JO, Weisenburger DD (1998) New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 16:2780–2795
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282. doi:10.1182/blood-2003-05-1545
Cheson BD, Horning SJ, Coiffier B et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol 17:1244
Atkinson AC (1980) A note on the generalized information criterion for choice of a model. Biometrika 67:413–418
Therneau TM, Grambsch PM (2000) Modeling survival data: exteding the Cox model. Springer, New York
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariate prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61:92–105. doi:10.1111/j.0006-341X.2005.030814.x
Gerds TA, Schumacher M (2006) Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biometrical J Biometrische Zeitschrift 48:1029–1040
Mogensen UB, Ishwaran H, Gerds TA (2012) Evaluating random forests for survival analysis using prediction error curves. J Stat Softw 50:1–23
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD (2001) Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54:774–781
Menter T, Ernst M, Drachneris J et al (2014) Phenotype profiling of primary testicular diffuse large B-cell lymphomas. Hematol Oncol 32:72–81. doi:10.1002/hon.2090
Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F (2013) WHO classification of tumors of soft tissue and bone, 4th edn. IARC, Lyon, France
Mozos A, Ye H, Chuang WY et al (2009) Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod Pathol 22:1210–1217. doi:10.1038/modpathol.2009.87
Kim YR, Kim JS, Min YH et al (2012) Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the consortium for improving survival of Lymphoma (CISL). J Hematol Oncol 5:49. doi:10.1186/1756-8722-5-49
Lee WJ, Won KH, Won CH et al (2015) Secondary cutaneous diffuse large B-cell lymphoma has a higher international prognostic index score and worse prognosis than diffuse large B-cell lymphoma. Leg Type Acta Derm Venereol. doi:10.2340/00015555-2139
Villa D, Connors JM, Sehn LH, Gascoyne RD, Savage KJ (2011) Diffuse large B-cell lymphoma with involvement of the kidney: outcome and risk of central nervous system relapse. Haematologica 96:1002–1007. doi:10.3324/haematol.2011.041277
Kim SJ, Kang HJ, Kim JS et al (2011) Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood 117:1958–1965. doi:10.1182/blood-2010-06-288480
Hwang HS, Yoon DH, Suh C, Park CS, Huh J (2014) Intestinal diffuse large B-cell lymphoma: an evaluation of different staging systems. J Korean Med Sci 29:53–60. doi:10.3346/jkms.2014.29.1.53
Moons KG, Kengne AP, Woodward M et al (2012) Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 98:683–690. doi:10.1136/heartjnl-2011-301246
Acknowledgments
This study was partly supported by a grant (2015-090) from the Asan Institute for Life Sciences, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board of the Asan Medical Center, Seoul, Korea.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hwang, H.S., Yoon, D.H., Suh, C. et al. A new extranodal scoring system based on the prognostically relevant extranodal sites in diffuse large B-cell lymphoma, not otherwise specified treated with chemoimmunotherapy. Ann Hematol 95, 1249–1258 (2016). https://doi.org/10.1007/s00277-016-2689-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2689-8