Abstract
Flow cytometric analysis of GPI-anchored proteins (GPI-AP) is the gold standard for diagnosis of paroxysmal nocturnal hemoglobinuria (PNH). Due to therapy options and the relevance of GPI-deficient clones for prognosis in aplastic anaemia detection of PNH is gaining importance. However, no generally accepted standard has been established. This study analysed the usefulness of a flow cytometric panel with CD58, CD59 on reticulocytes and erythrocytes, CD24/CD66b and CD16, FLAER on granulocytes and CD14, and CD48 on monocytes. Actual cut-off (mean + 2 SD) for GPI-deficient cells was established in healthy blood donors. We studied 1,296 flow cytometric results of 803 patients. Serial monitoring was analysed during a median follow-up of 1,039 days in 155 patients. Of all, 22% and 48% of 155 follow-up patients. showed significant GPI-AP-deficiency at time of initial analyses. During follow-up in 9%, a new PNH diagnosis, and in 28%, a significant change of size or lineage involvement was demonstrated. Highly significant correlations for GPI-AP deficiency were found within one cell lineage (r 2 = 0.61–0.95, p < 0.0001) and between the different cell lineages (r 2 = 0.49–0.88, p < 0.0001). Especially for detection of small GPI-deficient populations, reticulocytes and monocytes proved to be sensitive diagnostic tools. Our data showed superiority of reticulocyte analyses compared with erythrocyte analyses due to transfusion and hemolysis independency especially in cases with small GPI-deficient populations. In conclusion, a screening panel of at least two different GPI-AP markers on granulocytes, erythrocytes, and reticulocytes provides a simple and rapid method to detect even small GPI-deficient populations. Among the markers in our panel, CD58 and CD59 on reticulocytes, CD24/66b, and eventually FLAER on granulocytes as well as CD14 on monocytes were most effective for flow cytometric diagnosis of GPI deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hematopoietic stem cell disease [1, 2]. Pathophysiologically, it is based on somatic mutations of the X-linked phosphatidyl-inositol-glycan gene [3, 4] which result in a partial or absolute deficiency of GPI-linked proteins [5]. The lack of GPI-linked proteins leads to the clinical features chronic intravascular hemolytic anaemia and thromboembolism [31, 32]. The role of GPI-AP in bone marrow failure which is often associated with PNH is less clear [6].
Measurement of GPI-anchored proteins expression by flow cytometry is nowadays the gold standard for laboratory diagnosis of PNH and has replaced the Ham test and the sucrose lysis test (sugar water test) [7, 8]. Flow cytometry allows a sensitive and specific detection of even small GPI-deficient populations and a quantification of PNH cell populations in various cell lineages. This quantification is especially important, as the estimated size and type of the involved cell lineages correlates with different clinical manifestations of the disease [14]. For instance, patients with more than 20% type III deficiency on red cells mostly have clinically significant hemolysis, while nearly half of patients with more than 50% GPI deficiency on the granulocytes suffer from venous thrombosis in the first 10 years after initial diagnosis [15]. Therefore, the size of the GPI-AP-deficient population on granulocytes is a prognostic factor for vascular events, which have been shown to be the key determinant of prognosis with the most important impact on life expectancy. Remarkably, clone size and cell lineage involvement can change over time [6, 12, 21]. This leads to the need of follow-up investigations to initiate appropriate therapeutic approaches. Beside initial diagnosis, flow cytometry in particular, enables serial monitoring during the course of the disease [9–12]. This allows ontime determination of therapeutic decisions like initiation of prophylactic anticoagulation [15] or application of the C5-antibody eculizumab, which has already proven to reduce hemolytic activity and thromboembolic events [16–19]. Additionally, therapeutic effects may be monitored via flow cytometric analysis of GPI deficiency. Disappearance of the GPI-deficient populations indicates effective therapy after stem cell transplantation, while increase of the GPI-deficient erythrocyte populations upon eculizumab therapy is presumable due to a decrease of hemolytic activity with the result of a prolonged red blood cell (RBC) survival [18]. Although flow cytometric analysis of GPI-AP is the gold standard for diagnosis of PNH for years, there are no detailed international or even nationwide rules to determine minimal demands for PNH diagnosis based on flow cytometry. There exists just a general advice of the International PNH Interest Group (IPIG) to examine at least erythrocytes and granulocytes for diagnosis of PNH [21]. No consensus recommendations are available for the choice of GPI-AP markers or even the cell populations which should be examined. Thus, results from different laboratories are often difficult to compare and false-negative or false-positive results are not a rare event. Moreover, most tests are influenced by hemolytic crises or RBC transfusions as they rely on GPI deficiency of erythrocytes. Therefore, in this study, we retrospectively analysed the results of our routine diagnostics of the last 5 years to assess the most suitable GPI markers and cell populations for a sensitive, valid, and rapid GPI-AP flow cytometric analysis for diagnosis and serial testing.
Design and methods
Patients
Flow cytometric GPI-AP analysis was performed in 803 patients suffering from PNH or suspected GPI-deficient populations. The reason for flow cytometric analysis as reported by the treating physician were the following diagnoses or suspected diagnoses: PNH in 67 patients, hemolysis in 77 patients, aplastic anaemia or trilineage cytopenia in 291 patients, MDS in 44 patients, single lineage or bilineage cytopenia in 76 patients, unclear thromboembolic events or ischemias in 49 patients, other hematologic diseases in 25 patients, and insufficient information in 174 patients. In the majority of the patients, blood was sent for routine diagnostic assessment to our reference diagnostic laboratory at the Institute of Clinical Transfusion Medicine and Immunogenetics Ulm for analysis of GPI deficiency as a diagnostic routine procedure in patients with bone marrow failure, hemolytic anaemia, iron deficiency anaemia, thrombosis, or abdominal pain of unknown origin. Some of the analyses were performed as diagnostic test in clinical trials after informed consent and ethical committee approval of the trial. Patients were included in the follow-up analysis if at least two GPI-AP assessments with a minimum interval of 3 months were available (n = 155 patients). At time of initial GPI-AP analysis, blood count was available in 89 of the 155 follow-up patients; in this patients group, 48/89 patients showed neutropenia with <0.5 G/l and 70/89 patients had thrombopenia with <100 G/l. Patients with eculizumab treatment were censored at the date of first eculizumab application, and GPI-AP assessment of these patients during eculizumab therapy were not included in the analyses presented here. Five patients who received allogeneic stem cell transplantation were not censored for analysis.
The pattern of lineage involvement and the suitability of various lineages and markers in the peripheral blood for detection of GPI-deficient populations were statistical analysed retrospectively in all patients. For follow-up, the size of GPI-deficient populations as well as the evolution of lineage involvement was investigated.
Two-color flow cytometry
Immunophenotyping was performed as previously described [12]. Flow cytometric examinations (1,296) performed between March 2003 and August 2008 were analysed. Peripheral blood (PB) was obtained by venous puncture using Na-EDTA or citrate as anti-coagulant. All incubations were performed at room temperature in the dark. We used the flow cytometric analysis of CD58 and CD59 on reticulocytes and erythrocytes and of CD66b/CD24 and CD16 on granulocytes as mandatory markers for our screening panel. The expressions of CD14 and CD48 on monocytes as well as CD48/CD19 and CD48/CD3 on lymphocytes were used as optional markers to fully assess lineage involvement. In 584 cases, a combination of CD66b/CD24, CD16, and FLAER [23], a fluorochrome-labelled modified bacterial toxin which binds directly to the GPI anchor was used to confirm the diagnosis. Staining of the reticulocytes by the RNA dye thiazol orange allowed a separate analysis of erythrocytes and reticulocytes.
Staining of erythrocytes and reticulocytes (no wash, stain, no lyse protocol)
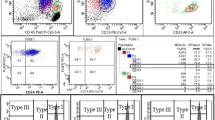
A volume of 100 μL of 1:10 diluted PB in PBS without Ca2+/Mg2+ (PAA Laboratories, Paching, Austria) was used for each assay. As isotype control, diluted PB was incubated with 20 μL of IgG*PE, clone ×40 (BD Immunocytometry Systems, Heidelberg, Germany). Detection of GPI-deficient erythrocytes and reticulocytes was performed by staining 100 μL of diluted PB with either 20 μL of CD58*PE, clone AICD58 (Beckman Coulter Immunotech, Krefeld, Germany) or 20 μL of CD*59 PE, clone p282 (H19) (BD Pharmingen, Heidelberg, Germany). After 20 min, cells were washed once by addition of 1 mL PBS without Ca2+/Mg2+ followed by centrifugation at 5,900×g for 0.5–1 min. The cells of all three assays were resuspended in 1 mL of ReticCOUNT Reagent (BD Immunocytometry Systems) and incubated for 20 min–1 h to stain RNA of reticulocytes before analysis by flow cytometry (Fig. 1).
Staining of the reticulocytes by the RNA dye thiazol orange allowed a separate analysis of erythrocytes and reticulocytes. Comparison of the results in a healthy control and a PNH patient. In the healthy control, separation of two populations according to erythrocytes (left upper quadrant) and reticulocytes (right upper quadrant) without significant GPI deficiency. In the PNH patient, separation of four populations according to erythrocytes and reticulocytes without (upper quadrants) and with (lower) significant GPI deficiency
Staining of granulocytes, monocytes (no wash, stain, lyse protocol)
To consider the contribution of different autofluorescences, separate isotype controls were performed for granulocytes and monocytes. Therefore, two assays of PB, containing 0.8 × 106–1.6 × 106 leukocytes each, were incubated with 20 μL of IgG*FITC and 20 μL of IgG* PE clone ×40 (both BD Immunocytometry Systems). Analysis of granulocytes was performed by staining PB with 10 μL of CD24*FITC, clone SN3 (DAKO, Hamburg, Germany), 10 μL of CD66b*FITC, clone 80H3 (Beckman Coulter Immunotech), and 20 μL of CD11b*PE, clone D12 (BD Immunocytometry Systems). In a separate assay, the corresponding amount of PB was incubated with 10 μL of CD16*FITC, clone NKP15 and 20 μL of CD11b*PE, clone D12 (both BD Immunocytometry Systems). For analysis of monocytes, PB was incubated either with 20 μL of CD14*FITC, clone MOP9 and 20 μL of CD33*PE, clone P67.6 (both BD Immunocytometry Systems) or 20 μL of CD48*FITC, clone J4.57 (Beckman Coulter Immunotech) and 20 μL*CD33, clone P67.6 (BD Immunocytometry Systems), respectively. After 20–30 min, cells were washed once by addition of 1 mL PBS without Ca2+/Mg2+ and centrifugation by 5,900×g for 0.5–1 min. Lysis of erythrocytes was performed by resuspension of the cell pellets in 100 μL OptiLyse B (Beckman Coulter Immunotech), incubation for 7–15 min, addition of 1 mL aqua followed by vigorous mixing, and incubation for another 5–15 min. After lysis of erythrocytes, cells were washed twice using 1 mL PBS without Ca2+/Mg2+ and resuspended 300–800 μL PBS without Ca2+/Mg2+ for flow cytometry analysis.
Staining of granulocytes (wash, lyse, stain protocol)
For detection of GPI-deficient granulocytes by FLAER (FITC labeled aerolysin, Protox Biotech, Victoria, Canada), a volume of PB blood containing approximately 2 × 105 neutrophils was washed with the fivefold volume of PBS without Ca2+/Mg2+, and the supernatant was discarded. Erythrocytes were lysed as described above using half the volume of OptiLyse B and aqua and washed twice, using PBS without Ca2+/Mg2+. Half of the remaining cells were stained with 2–5 μL of a 1:10 dilution of IgG*PE, clone ×40 (BD Immunocytometry Systems) in PBS, the other half of the cells was stained with a 1:50 dilution of CD16*PE, clone 3G8 (BD Pharmingen) in PBS and 0.5–5 μL FLAER, respectively. After 20–30 min, 1 mL of PBS without Ca2+/Mg2+ was added, supernatant was removed after centrifugation at 5,900×g for 30 s–1 min, and cells were resuspended in 300–800 μL of PBS without Ca2+/Mg2+.
Normal range—cut off values of GPI deficiency
In the 5-year observation period every week, healthy blood donors (overall n = 268) were tested for GPI deficiency to establish cut-off values (mean + 2 SD) for GPI-deficient cells. In respect of variabilities in antibody efficiency, we updated this cut-off every 6 months with the results of the last 50–60 examined healthy donors. The valid cut-offs at the end of the 5-year observation period (overall results of the 268 healthy donors were put in parentheses) were: reticulocytes CD58, 0.5% (1.0%), reticulocytes CD 59, 1.9% (2.0%), erythrocytes CD58, 0.1% (0.2%), erythrocytes CD59, 0.5% (0.8%), granulocytes CD66b/CD24, 0.1% (0.7%), granulocytes CD16, 1.0 % (1.2%), granulocytes FLAER*,CD16, 0.3 % (1.2%); monocytes CD14, 2.5 % (3.3%), monocytes CD48, 1.1% (1.1%).
For flow cytometric diagnosis of a PNH cell population, at least two cell lineages with significant GPI-deficient populations were required. Significant GPI-deficient populations are defined if all examined markers (minimum, 2) were exceeding the cut-off value.
Statistical analysis
Statistical analyses of the data were carried out in Microsoft Office Access 2003 and GraphPad PRISM, version 4.00. Used tests were linear regression, including correlations, coefficient of correlation, and confidence interval as well as scatter plots. A p value of less than 0.05 was considered as statistically significant.
Results
Patient characteristics
Patient characteristics for the examined 803 patients (416 male; 382 female) were as follows: the patient age ranged from 0.4 to 90.7 years (median age, 32.4 years) with no relevant differences between men (52%) and women (48%) (Table 1). At initial GPI-AP flow cytometry, 176 of all patients (22%) were diagnosed with PNH typical GPI-deficient populations (Fig. 2). The groups with and without PNH diagnosis at time of initial GPI-AP analysis do not differ significantly in age and sex distribution. For detailed patient characteristics, see Table 1.
Flow sheet patient of the different patient groups at time of initial GPI-AP analysis and during follow-up. Significant changes of GPI-deficient populations during follow-up was observed in groups B, D1, D2 with an expansion of GPI-AP-deficient populations (including new PNH diagnosis) and in groups E1, E2, and F with a decrease of GPI-AP-deficient populations (including disappearance of PNH diagnosis criteria)
In the follow-up cohort, 155 patients and 625 flow cytometric analyses were evaluated (median number of analyses per patient, 3; range from 2 to 26) for a median follow-up duration of 1,039 days. Patient characteristics of the follow-up group are summarised in Table 1.
Blood count was evaluable in 89 of these patients at the time of initial assessment and in 120 patients at time of the last examination. Thrombocytopenia < 100 G/l was obtained in 62% at the initial examination and in 43% at the last examination. Leukocytopenia < 0.5 G/l was initially obtained in 36% and decreased to 18% at the final investigation (see data supplements).
Flow cytometric results
Analysis of different GPI-anchored markers in the same cell lineage
Primarily, we compared all flow cytometric results (including the follow-up analyses) within each separate cell lineage to assess the correlation of the various GPI-linked markers. We found a highly significant correlation between the markers CD58 and CD59 on reticulocytes (r 2 = 0.95, p < 0.0001; n = 1,284) and erythrocytes (r 2 = 0.86, p < 0.0001; n = 1,284), between CD24/66b and CD16 or between CD24/66b and CD16, FLAER on granulocytes (r 2 = 0.76, p < 0.0001; n = 1248), and between CD14 and CD48 on monocytes (r 2 = 0.62, p < 0.0001; n = 599) (Fig. 3). If we restricted the analysis to the first GPI-AP flow cytometric analysis in each patient, the correlation did not change considerably (Fig. 3).
Correlation between the used GPI-AP markers within one cell line. Black = all analyses. Red = restricted to the first analysis per patient. Broken black lines = confidence interval of all analyses. Broken red lines = confidence interval of the first analysis per patient. All correlations were highly significant (p < 0.0001) in the group with all analyses as well as in the group with only the first analysis (p < 0.0001). The range of the correlation coefficient was r 2 = 0.61–0.95
Marker analysis between different cell lineages
In a second step, we compared the sizes of various GPI-marker negative populations in different cell lineages. This analysis was initially performed for all GPI-AP flow cytometric results in patients with flow cytometric diagnosis of PNH. The following significant correlations were obtained: CD58-negative reticulocytes and CD58-negative erythrocytes (n = 457, r 2 = 0.63, p < 0.0001), CD59-negative reticulocytes and CD59-negative erythrocytes (n = 455, r 2 = 0.61, p < 0.0001), CD59-negative reticulocytes and CD24/D66b-negative granulocytes (n = 450, r 2 = 0.60, p < 0.0001), CD59-negative reticulocytes and CD14-negative monocytes (n = 381, r 2 = 0.68, p < 0.0001), CD59-negative erythrocytes and CD24/66b-negative granulocytes (n = 449, r 2 = 0.33, p < 0.0001), CD14-negative monocytes and CD24/66b-negative granulocytes (n = 423, r 2 = 0.84, p < 0.0001), CD48-negative monocytes and CD24/66b-negative granulocytes (n = 421, r 2 = 0.48, p < 0.0001) (Fig. 4).
Correlation between the used GPI-AP markers on the different cell lines. Black = all analyses. Red = restricted to the first analysis per patient. Broken black lines = confidence interval of all analyses. Broken red lines = confidence interval of the first analysis per patient. All correlations were highly significant in the group with all analyses (p < 0.0001) as well as in the group with only the first analysis (p < 0.0001). The range of the correlation coefficient was r 2 = 0.33 up to 0.88. The lowest value for r 2 (r 2 = 0.33) was observed between CD59 on erythrocytes and CD24/CD66b on granulocytes, whereas the correlation between CD59 on reticulocytes and CD24/CD66b on granulocytes was r 2 = 0.60
In a second step, the analysis was restricted to the first flow cytometric analysis of each patient with flow cytometric diagnosis of PNH. There were no significant differences between both analyses (Fig. 4).
Marker analysis for detection of small GPI-deficient populations
To elucidate the importance of individual markers for the flow cytometric diagnosis of PNH, all markers were separately analysed. Depending on the size of the CD24/CD66b-negative granulocyte population, we defined two different subgroups based on a CD24/CD66b-negative-granulocyte population < 10% or ≥10% at the first investigation. The largest median GPI-deficient population in the subgroup with <10% CD24/66b-negative granulocyte populations was observed for CD14 on monocytes, CD16 or CD16, FLAER on granulocytes, and CD59 on reticulocytes. In the subgroup, with ≥10% CD24/CD66b-negative-granulocyte population, the largest median GPI-deficient population was shown for CD14 on monocytes, CD24/66b and CD16 as well as CD16, FLAER on granulocytes, and CD59 on reticulocytes (Fig. 5).
Scatter blots with marker deficient cells in percent in patients with ≥10% GPI-deficient granulocyte population (B + D) and in patients with <10% GPI-deficient granulocyte population (A + C). ECD58 = CD58-deficiency on erythrocytes, ECD59 = CD59 deficiency on erythrocytes, RCD58 = CD58 deficiency on reticulocytes, RCD59 = on reticulocytes, GCD24/66b = CD24/66b-deficiency on granulocytes, GFLAER or GCD16 = CD16 and/or FLAER or CD16-deficiency on granulocytes, MCD14 = CD14-deficiency on monocytes, MCD48 = CD48 deficiency on monocytes. Blue line median size of GPI-deficient population
Follow-up analysis
We analysed repeated measurements of GPI-AP expression on reticulocytes, erythrocytes, granulocytes, and monocytes in 155 patients (Fig. 6) in order to study the dynamics of GPI-AP deficient cell populations over time. Since it has been demonstrated that treatment with eculizumab changes, the relative proportion of GPI-AP deficient red cells [19], patients who received eculizumab treatment were censored at start of this treatment. Median number of analyses per patient was 3 and the median follow-up duration was 1,039 days (minimum 90 days, maximum 1,903 days). Initially, 75 (48%) of these patients were diagnosed with PNH. The longitudinal studies revealed different patterns of GPI-AP expression during the follow-up period (Figs. 2, 6, and 7): (A) no significant GPI-AP deficiency fulfilling our criteria for flow cytometric PNH diagnosis during the whole investigation period; (B) new flow cytometric diagnosis of PNH during follow-up after a normal GPI-AP expression at first investigation; (C) flow cytometric diagnosis of PNH at initial analysis with stable size of GPI-deficient granulocyte population during follow-up (defined as GPI-deficient granulocyte population stable <50% or ≥50%); (D) flow cytometric diagnosis of PNH at initial analysis with expansion of the GPI-deficient granulocyte population (defined as increase of GPI-deficient granulocyte population from <50% to ≥50%) (D1) and/or involvement of additional cell lineages (D2) during follow-up; (E) flow cytometric diagnosis of PNH at initial analysis and decrease of the GPI-deficient granulocyte population (defined as decrease of GPI-deficient granulocyte population from ≥50% to <50%); (E1) or disappearance of GPI deficiency in a cell lineage (E2) during longitudinal analysis; (F) disappearance of the PNH typical GPI-AP deficiency below our criteria for flow cytometric PNH diagnosis. The cut-off granulocyte clone size of </≥50% as sign of a relevant quantitative change in clone size was chosen because of published data about the impact of this relative proportion for thromboembolic events [15]. Patient characteristics, number of examinations, and duration of follow-up are summarised in Table 1. In detail, 9% of the 80 patients with no PNH typical GPI-AP deficiency in the first analysis developed flow cytometric PNH diagnosis during follow-up (group B). In three of these seven cases detected during follow-up, diagnosis was based on significant GPI-AP-deficient populations on monocytes and granulocytes. Of the follow-up patients, 28% showed a significant change of GPI-AP deficiency on granulocytes or lineage involvement. Moreover, 5 out of the 75 patients initially diagnosed with PNH developed an emerging GPI-deficient population on an additional cell lineage or showed less involved cell lineages during follow-up (group D2 and E2). In 11% of all cases with flow cytometric diagnosis of PNH, a significant change of GPI-deficient granulocyte population occurred (group D1 and E1). In the group of the seen patients with decrease of GPI-deficient granulocyte population or disappearance of significant GPI-AP deficiency in a cell line (E1 and E2), two patients received allogeneic stem cell transplantation, and two patients were treated with ATG-based immunosuppressive therapy. Only three of these seven patients suffered from a classical PNH, while four were diagnosed with associated bone marrow failure syndromes. All three patients with classical PNH showed a decrease of GPI-AP deficient population on granulocytes and no change in cell lineage involvement. Additionally, eight patients (11%) fulfilled no longer the flow cytometric criteria of PNH during follow-up (group F). Half of these eight patients received an ATG-based immunosuppression and four patients (one was treated for MDS) received an allogeneic stem cell transplantation before the PNH typical GPI-AP deficient population pattern disappeared. A spontaneous loss of significant GPI deficiency clones, i.e., disappearance without prior therapy, was observed in none of the follow-up patients. All such cases were obtained in association with therapy response and blood count improvement.
Examples of follow-up courses for each follow-up subgroup A–F. a Flow cytometric criteria for PNH at initial diagnosis and during follow-up not fulfilled; b New flow cytometric diagnosis of PNH during follow-up. c Stable GPI deficiency during follow-up. d Increase of GPI deficiency during follow-up. e Decrease of GPI deficiency during follow-up. f Flow cytometric diagnosis criteria for PNH lost during follow-up
Scatter blots of GPI-marker deficiency in percent during follow-up. ECD59 = CD59 deficiency on erythrocytes, RCD59 = on reticulocytes, GCD24/66b = CD24/66b deficiency on granulocytes, MCD14 = CD14 deficiency on monocytes. Blue line: median size of GPI-deficient population. First examination = results of group B (no flow cytometric PNH diagnosis at first GPI-AP-analysis, but fulfilling flow cytometric PNH diagnosis criteria during follow-up) at time of initial GPI-AP analysis. Initial diagnosis = results of group C + D + E + F (flow cytometric PNH diagnosis at first GPI-AP analysis) at time of first GPI-AP-analysis and results of group B at time of first flow cytometric PNH diagnosis. Last follow-up: results of all patients with flow cytometric PNH diagnosis at time of last GPI-AP analysis
Discussion
Flow cytometry of GPI-AP is considered to be the gold standard of PNH diagnosis. CD55 and CD59 expression on erythrocytes are most frequently used for screening of PNH [6, 7, 9–14, 21, 22]. Our presented data show that addition of further antibodies against GPI-linked antigens on different cell lineages considerably improves the sensitivity and validity of the method. Among the markers evaluated, we recommend a panel including at least the following markers: CD58 and CD59 for reticulocytes and erythrocytes a combination of CD24/66b and eventually FLAER on the granulocytes and CD14 on monocytes.
It has been shown that detection of GPI-deficient reticulocytes is more sensitive than analysis of erythrocytes, and reticulocytes better correlates with proportion of GPI-deficient white cells. The remarkable worse correlation between erythrocytes and granulocytes compared to the correlation of reticulocytes and granulocytes (Fig. 4) and the larger GPI-deficient populations in reticulocyte than erythrocyte (Fig. 5) are probably caused by RBC transfusions, hemolytic crises, and a shorter life span of PNH erythrocytes [24, 25]. As reticulocytes are less affected by these events, their examination for GPI-AP deficiency is especially suitable for diagnosis of involvement of the red cell lineage if there are small GPI-deficient granulocyte populations below 10%. Nevertheless, the most international recommendations for flow cytometry still favour erythrocyte analysis. Our data emphasise the importance of reticulocyte analysis. Even if reticulocyte clone size correlates well with white cell clone size, both should be tested to confirm the flow cytometric diagnosis of PNH because alteration of GPI-AP on leukocytes can be a sign of granulocytic/monocytic dysplasia and immaturity ([30], data supplements) in the context of various myeloid diseases or cytolysis. Due to our results, participation of leukocytes is detected best by examination of CD14-deficiency on monocytes (Fig. 5). Therefore, additional examination of monocytes provides a higher detection sensitivity of GPI-AP deficiency especially in patients with small GPI-deficient granulocyte populations <10%. In fact, the marker combination CD24/66b and CD16 seems to be suitable in sensitivity and validity to detect GPI-deficient granulocytes in our analysis.
Follow-up examination is recommended by the International PNH Interest Group (IPIG) in case of bone marrow failure syndromes [21] even in case of initially normal flow cytometric analysis. Moreover, regular follow-up studies are indicated in case of known PNH to detect substantial changes in GPI-AP-deficient population size or cell lineage involvement, as both situations may prompt therapeutic consequences or may demonstrate effect of therapeutic interventions. Therefore, we evaluated our panel on different cell lineages for longitudinal monitoring of GPI-deficient populations to determine the frequency of significant changes in cell lineage involvement and GPI-deficient population size. Our definition of a relevant change in GPI-deficient population size (</≥50% GPI deficiency on granulocytes) is based on data published by Hall et al. [15], demonstrating that patients with a GPI-deficient population on granulocytes ≥50% have a significant higher risk of thromboembolic events. Our results again support the importance of GPI-AP measurement on reticulocytes to obtain results independent of hemolytic crisis and RBC transfusions.
The described flow cytometry panel could be used for serial monitoring during follow-up to detect evolution of GPI-AP deficient populations and to assess therapy effects. Although follow-up investigations were performed only in a minority of patients with initially normal results (80 out of 627 patients), in seven of the follow-up patients, PNH was newly diagnosed. Based on our follow-up data with a relevant proportion of newly developing GPI-AP-deficient populations and significant changes in the size of GPI-AP-deficient populations, repeated GPI-AP analysis in regular intervals should be performed, like it is recommended by the International PNH Interest Group (IPIG) [21]. Another interesting fact in our follow-up data is the absence of spontaneous remissions of PNH typical GPI-AP-deficient populations which had been reported. A study with 35 patients referred a rate of 15% spontaneous remissions [6] in PNH patients, but available GPI-AP flow cytometric results and even HAM-test were limited in this study. All observed remissions in our patient group happened in the context of stem cell transplantation or intensified immunosuppressive therapy especially in patients with AA-PNH syndrome. The reported three patients with classical PNH and decrease of the GPI-AP deficient population on granulocytes below 50% developed an increase over 50% GPI-deficient cells on the granulocytes during further follow-up.
Generally, expression of GPI-anchored proteins should be analysed in all situations suspicious of PNH and in bone marrow failure syndromes [20]. According to our data, measurement of at least two different GPI-anchored proteins on granulocytes, erythrocytes, and reticulocytes provides a simple and rapid method to detect even small GPI-deficient populations. Measurement of erythrocytes only includes the pitfall of false-negative results or false low values of GPI-deficient populations. On the contrary, examination of GPI deficiency on reticulocytes additionally prevents false positive interpretation of significant GPI-deficient populations on the leukocytes (data supplements). GPI-AP deficiencies without clinical PNH were reported in the context of other hematologic diseases or after immunosuppressive therapy [26] as well as in healthy individuals [27, 28]. Our data demonstrate that in patients with small GPI-deficient populations, these can in particular be detected on monocytes. In conclusion, we recommend a flow cytometric screening panel with the markers CD58 and CD59 on reticulocytes and erythrocytes as well as with the markers CD24/66b and CD16 on granulocytes for initial diagnosis and monitoring during follow-up. In case of significant GPI-AP-deficient population in a minimum of one cell lineage, the analysis should be extended to the markers CD14 and CD48 on monocytes. Furthermore, we advise a serial monitoring for PNH patients as well as for patients with bone marrow failure syndromes without a present GPI-AP deficiency every 6 months or in case of significant clinical symptoms.
Recently, Richards et al. [29] showed that a relevant proportion of laboratories doing PNH testing by flow cytometry have significant problems with regard to false-positive and false-negative results. On the other hand, due to the advent of a new targeted therapy option, early diagnosis and serial monitoring of PNH is gaining importance for a better patient management [17]. As a consequence, there is the urgent need for optimised flow cytometric protocols in PNH diagnosis. In conclusion, this analysis has demonstrated that the described flow cytometric method offers significant benefits in sensitivity and validity in PNH testing and can be therefore recommended for a wider use.
References
Dacie JV (1963) Paroxysmal nocturnal haemoglobinuria. Proc R Soc Med 56:587–596
Oni SB, Osunkoya BO, Luzzatto L (1970) Paroxysmal nocturnal hemoglobinuria: evidence of monoclonal origin of abnormal red cells. Blood 36:145–152
Myata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, Maeda K, Kitani T, Kinoshita T (1993) The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science 259:1318–1320
Mortazavi Y, Merk B, McIntosh J, Marsh JC, Schrezenmeier H, Rutherford TR, BIOMED II Pathophysiology and Treatment of Aplastic Anaemia Study Group (2003) The spectrum of PIG-A mutations in aplastic anemia/paroxysmal nocturnal hemoglobinuria: a high incidence of multiple mutations and evidence of a mutational hot spot. Blood 101(7):2833–2841
Bessler M, Mason PJ, Hillmen P, Miyata T, Yamada N, Takeda N, Luzzatto L, Kinoshita T (1994) Paroxysmal nocturnal hemoglobinuria is caused by somatic mutations in the PIG-A gene. EMBO J 13:110–117
Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV (1995) Natural history of paroxysmal nocturnal hemoglobinuria. N Eng J 333:1253–1258
Hillmen P, Hows JM, Luzzatto L (1992) Two distinct patterns of glycosylphosphatidylinositol (GPI) linked protein deficiency in the red cells of patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 80(3):399–405
de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, Socie G (2008) Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood 112:3099–3106
Hernandez-Campo PM, Almeida J, Acevedo MJ, Sanchez ML, Alberca I, Vidriales B, Martinez E, Romero JR, Orfao A (2008) Detailed immunophenotypic characterization of different major and minor subsets of peripheral blood cells in patients with paroxysmal nocturnal hemoglobinuria. Transfusion 48:1403–1414
Richards SJ, Hillmen P (2002) Immunophenotypic analysis of PNH cells. Curr Protoc Cytom, Chapter 6:Unit 6.11
Nebe T, Schubert J, Gutensohn K, Schrezenmeier H (2003) Flow cytometric analysis of GPI-deficient cells for the diagnosis of paroxysmal nocturnal hemoglobinuria (PNH). J Lab Med 27:257–265
Schrezenmeier H, Hildebrand A, Rojewski M, Hacker H, Heimpel H, Raghavachar A (2000) Paroxysmal nocturnal haemoglobinuria: a replacement of haematopoietic tissue? Acta Haematol 103:41–48
Moyo VM, Mukhina GL, Garrett ES, Brodsky RA (2004) Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol 126:133–138
Richards SJ, Barnett D (2007) The role of flow cytometry in the diagnosis of PNH in the clinical laboratory. Clin Lab Med 27:577–590
Hall C, Richards S, Hillmen P (2003) Primary prophylaxis with warfarin prevents thrombosis in PNH. Blood 102:3587–3591
Hillmen P, Muus P, Dührsen U, Risitano A, Schubert J, Luzzatto L, Schrezenmeier H, Jeffrey S, Brodsky RA, Hill A, Socie G, Bessler M, Rollins S, Bell L, Rother R, Young NS (2007) Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysman nocturnal hemoglobinuria. Blood 110(12):4123–4128
Schrezenmeier H, Höchsmann B (2009) Eculizumab opens a new era of treatment for paroxysmal nocturnal hemoglobinuria. Expert Rev Hematol 2(1):7–16
Hill A, Richards SJ, Hillmen P (2007) Recent developments in the understanding and management of PNH. Br J Haematol 137:181–192
Hill A, Hillmen P, Richards SJ et al (2005) Sustained response and long term safety of eculizumab in PNH. Blood 106:2559–2565
Schrezenmeier H, Hertenstein B, Wagner B et al (1995) A pathogenetic link between aplastic anemia and paroxysmal nocturnal haemoglobinuria is suggested by a high frequency of aplastic anemia patients with a deficiency of phophatidylinositol glycan anchored proteins. Exp Hematol 23:81–87
Parker C, Omine M, Socie G et al (2005) Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 106(12):3699–3709
Hall SE, Rosse WF (1996) The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood 87:5332–5340
Brodsky RA, Mukhina GL, Li S et al (2000) Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol 114:459–466
Rosse WF, Dacie JV (1966) Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. J Clin Invest 45(5):736–745
Navenot JM, Muller JY, Blanchard D (1998) Investigation of the survival of paroxysmal nocturnal hemoglobinuria red cells through the immunophenotyping of reticulocytes. Transfusion 38(4):337–342
Taylor VC, Sims M, Brett S, Field MC (1997) Antibody selection against CD52 produces a paroxysmal nocturnal haemoglobinuria phenotype in lymphocytes by a novel mechanism. Biochem J 322:919–925
Young NS (1992) The problem of clonality in aplastic anemia: Dr Dameshek's riddle, restated. Blood 79(6):1385–1392
Bessler M, Mason P, Hillmen P, Luzzatto L (1994) Somatic mutations and cellular selection in paroxysmal nocturnal haemoglobinuria. Lancet 343(8903):951–953
Richards SJ, Whitby L, Cullen MJ, Barnett D et al (2009) Development and evaluation of a stabilized whole-blood preparation as a process control material for screening of paroxysmal nocturnal hemoglobinuria by flow cytometry. Cytom B Clin Cytom 76 B:47–55
Wang SA, Pozdnyakova O, Jorgensen JL, Medelros J, Stachurski D, Anderson M, Raza A, Woda BA (2009) Detection of paroxysmal nocturnal hemoglobinuria clones in patients with myelodysplastic syndromes and related bone marrow diseases, with emphasis on diagnostic pitfalls and caveats. Haematologica 94(1):29–37
Helley D, de Latour RP, Porcher R, Rodrigues CA, Galy-Fauroux I, Matheron J, Duval A, Schved JF, Fischer AM, Socié G, French Society of Hematology (2010) Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica 95(4):574–581
Hill A, Rother RP, Wang X, Morris SM Jr, Quinn-Senger K, Kelly R, Richards SJ, Bessler M, Bell L, Hillmen P, Gladwin MT (2010) Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 149(3):414–425
Acknowledgments
The authors would like to thank Gisela Baur, Thomas Becker, and Rosi Leichtle for skilful technical and statistical evaluation assistance.
Authorship and disclosures
BH and HS took primary responsibility for the paper and designed research. BH and MR performed laboratory work. BH, HS, and MR analysed the data and wrote the paper.
BH and HS were advisors for and received honoria from Alexion. MR reported no potential conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
BH and HS contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 347 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Höchsmann, B., Rojewski, M. & Schrezenmeier, H. Paroxysmal nocturnal hemoglobinuria (PNH): higher sensitivity and validity in diagnosis and serial monitoring by flow cytometric analysis of reticulocytes. Ann Hematol 90, 887–899 (2011). https://doi.org/10.1007/s00277-011-1177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1177-4