Abstract
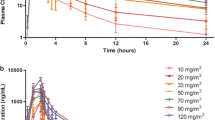
ITF2357, an orally effective member of the family of histone deacetylase inhibitors, is a potent inducer of apoptosis and death of multiple myeloma (MM) cells. We performed a phase-II, multiple-dose clinical trial in 19 patients with relapsed or progressive MM to determine the maximum tolerated dose (MTD) of ITF2357 administered twice daily for four consecutive days every week for 4 weeks (i.e., first cycle). The first six patients received 150 mg ITF2357 twice daily. Since two of them experienced a dose-limiting toxicity (DLT) during the first cycle, the subsequent patients received 100 mg ITF2357 twice daily. This was the MTD, as only one DLT occurred. Up to 12 weeks (i.e., three cycles) of treatment were scheduled. Oral dexamethasone was allowed to a maximum weekly amount of 20 mg. Median duration of treatment was 6 weeks, ranging from two (two patients) to 12 weeks (five patients). Four patients suffered from serious adverse events. Three patients experienced grade 3–4 gastro-intestinal toxicity and three had transient electrocardiographic abnormalities. Thrombocytopenia occurred in all but one patient (grade 3–4 in ten patients). At last follow-up, five patients were in stable disease, five had disease progression, and nine had died all of progressive MM. In conclusion, when given at a dose of 100 mg twice daily alone or combined with dexamethasone, ITF2357 proved tolerable but showed a modest clinical benefit in advanced MM.
Similar content being viewed by others
References
Alastair Smith FW (2006) Diana Samson, Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol 132:410–451. doi:10.1111/j.1365-2141.2005.05867.x
Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC (2004) Focus on multiple myeloma. Cancer Cell 6:439–444. doi:10.1016/j.ccr.2004.10.020
Richon VM, Webb Y, Merger R et al (1996) Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci USA 93:5705–5708. doi:10.1073/pnas.93.12.5705
Cohen LA, Amin S, Marks PA, Rifkind RA, Desai D, Richon VM (1999) Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiation agent, suberanilohydroxamic acid (SAHA). Anticancer Res 19:4999–5005
Butler LM, Agus DB, Scher HI et al (2000) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60:5165–5170
Kelly WK, O'Connor OA, Krug LM et al (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931. doi:10.1200/JCO.2005.14.167
O'Connor OA, Heaney ML, Schwartz L et al (2006) Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol 24:166–173. doi:10.1200/JCO.2005.01.9679
Garcia-Manero G, Yang H, Bueso-Ramos C et al (2008) Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 111:1060–1066. doi:10.1182/blood-2007-06-098061
Duvic M, Talpur R, Ni X et al (2007) Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109:31–39. doi:10.1182/blood-2006-06-025999
Olsen EA, Kim YH, Kuzel TM et al (2007) Phase IIB multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25:3109–3115. doi:10.1200/JCO.2006.10.2434
Richardson P, Mitsiades C, Colson K et al (2008) Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma 49:502–507
Badros A, Philip S, Niesvizky R et al (2007) Phase I trial of suberoylanilide hydroxamic acid (SAHA) + bortezomib (Bort) in relapsed multiple myeloma (MM) patients (pts). ASH Annual Meeting Abstracts 110:1168
Weber DM, Jagannath S, Mazumder A et al (2007) Phase I trial of oral vorinostat (Suberoylanilide Hydroxamic Acid, SAHA) in combination with bortezomib in patients with advanced multiple myeloma. ASH Annual Meeting Abstracts 110:1172
Kelly WK, Richon VM, O'Connor O et al (2003) Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 9:3578–3588
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12:1247–1252
Golay J, Cuppini L, Leoni F et al (2007) The histone deacetylase inhibitor ITF2357 has anti-leukemic activity in vitro and in vivo and inhibits IL-6 and VEGF production by stromal cells. Leukemia 21:1892–1900
Blade J, Samson D, Reece D et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European group for blood and marrow transplant. Br J Haematol 102:1115–1123
Barlogie B, Jagannath S, Desikan KR et al (1999) Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 93:55–65
Shaughnessy J Jr, Tian E, Sawyer J et al (2003) Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. Br J Haematol 120:44–52
Piekarz RL, Frye AR, Wright JJ et al (2006) Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res 12:3762–3773
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galli, M., Salmoiraghi, S., Golay, J. et al. A phase II multiple dose clinical trial of histone deacetylase inhibitor ITF2357 in patients with relapsed or progressive multiple myeloma. Ann Hematol 89, 185–190 (2010). https://doi.org/10.1007/s00277-009-0793-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-009-0793-8