Abstract
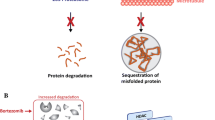
Oral panobinostat (Farydak®), a potent nonselective histone deacetylase inhibitor, is approved in several countries for use in combination with bortezomib and dexamethasone in patients with multiple myeloma (MM) [USA] or relapsed and/or refractory MM (EU) who have received at least two prior treatment regimens, including bortezomib and an immunomodulatory drug (IMiD). In a pivotal phase III trial (PANORAMA 1) in patients with relapsed or relapsed and refractory MM who had received one to three previous lines of therapy, progression-free survival (PFS) was significantly prolonged with panobinostat plus bortezomib and dexamethasone compared with placebo plus bortezomib and dexamethasone. The significantly favourable effect of panobinostat- versus placebo-based treatment on PFS was also observed in a subgroup analysis of patients who had previously received an IMiD, bortezomib plus an IMiD, or at least two lines of treatment including bortezomib and an IMiD. Panobinostat plus bortezomib and dexamethasone had a generally manageable tolerability profile, with the most frequent grade 3–4 adverse events being myelosuppression, diarrhoea, asthenia or fatigue, peripheral neuropathy and pneumonia. Thus, panobinostat, in combination with bortezomib and dexamethasone, is a useful addition to the available treatment options for patients with relapsed or refractory MM.

Similar content being viewed by others
References
Anderson KC, Alsina M, Atanackovic D. National Comprehensive Cancer Network clinical practice guidelines in oncology: multiplemyeloma, version 2.2016. 2015. http://www.nccn.org/patients. Accessed 11 Dec 2015.
Richardson PG, Laubach JP, Lonial S, et al. Panobinostat: a novel pan-deacetylase inhibitor for the treatment of relapsed or relapsed and refractory multiple myeloma. Expert Rev Anticancer Ther. 2015;15(7):737–48.
Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26(1):149–57.
Laubach JP, Voorhees PM, Hassoun H, et al. Current strategies for treatment of relapsed/refractory multiple myeloma. Expert Rev Hematol. 2014;7(1):97–111.
Torimoto Y, Shindo M, Ikuta K, et al. Current therapeutic strategies for multiple myeloma. Int J Clin Oncol. 2015;20(3):423–30.
Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280(2):233–41.
Mithraprabhu S, Kalff A, Chow A, et al. Dysregulated class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics. 2014;9(11):1511–20.
Richardson PG, Mitsiades CS, Laubach JP, et al. Preclinical data and early clinical experience supporting the use of histone deacetylase inhibitors inmultiplemyeloma. Leuk Res. 2013;37(7):829–37.
Novartis Pharmaceuticals Corporation. Farydak® (panobinostat) capsules, for oral use: US prescribing information. 2015. http://www.fda.gov. Accessed 11 Dec 2015.
Novartis Europharm Limited. Farydak hard capsules: EU summary of product characteristics. 2015. http://ema.europa.eu. Accessed 11 Dec 2015.
Novartis AG. Novartis Pharmaceuticals corporate fact sheet. 2015. http://www.novartis.com. Accessed 11 Dec 2015.
Rajkumar SV. Panobinostat for the treatment of multiple myeloma. Lancet Oncol. 2014;15(11):1178–9.
Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and alphatubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108(10):3441–9.
Maiso P, Carvajal-Vergara X, Ocio EM, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66(11):5781–9.
Ocio EM, Vilanova D, Atadja P, et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica. 2010;95(5):794–803.
San-Miguel JF, Richardson PG, Günther A, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013;31(29):3696–703.
San-Miguel JF, Hungria VTM, Yoon S-S, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractorymultiplemyeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–206.
Richardson PG, Hungria VTM, Yoon S-S, et al. Panobinostat plus bortezomib and dexamethasone in relapsed/relapsed and refractory myeloma: outcomes by prior treatment. Blood. 2015. doi:10.1182/blood-2015-09-665018.
Wolf JL, Siegel D, Goldschmidt H, et al. Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53(9):1820–3.
Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomibrefractory myeloma. Blood. 2013;122(14):2331–7.
Moreau P, San Miguel J, Ludwig H. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):vi133–7.
Niesvizky R, Ely S, Mark T, et al. Phase 2 trial of the histone deacetylase inhibitor romidepsin for the treatment of refractorymultiple myeloma. Cancer. 2011;117(2):336–42.
Richardson P, Mitsiades C, Colson K, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49(3):502–7.
US Food and Drug Administration. FDA approves Farydak for treatment of multiple myeloma [media release]. 23 Feb 2015. http://www.fda.gov.
Novartis AG. Novartis receives FDA approval of Farydak®, the first HDAC inhibitor for patients with multiple myeloma [media release]. 23 Feb 2015. http://www.novartis.com.
Novartis AG. Novartis receives EU approval for Farydak®, the first in its class of anticancer agents approved for patients with multiple myeloma [media release]. 4 Sep 2015. http://www.novartis.com.
Dimopoulos M, Siegel DS, Lonial S, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): a multicentre, randomised, doubleblind study. Lancet Oncol. 2013;14(11):1129–40.
Berdeja JG, Hart LL, Mace JR, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100(5):670–6.
Chari A, Cho HJ, Parekh S, et al. A phase II, single-center, open-label study of oral panobinostat in combination with lenalidomide and weekly dexamethasone in patients with multiple myeloma [abstract]. Blood. 2014;124(21):3486.
Popat R, Brown S, Flanagan LM, et al. Velcade, thalidomide, dexamethasone and panobinostat (VTD-P) for patients with relapsed and relapsed/refractory myeloma: preliminary results of the Muk-Six phase I/IIa trial [abstract]. Blood. 2014;124(21):4766.
Berenson JR, Hilger JD, Yellin O, et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann Hematol. 2014;93(1):89–98.
Offidani M, Polloni C, Cavallo F, et al. Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractorymultiplemyeloma. Leuk Lymphoma. 2012;53(9):1722–7.
Acknowledgments
During the peer review process, the manufacturer of panobinostat was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of Interest
Sarah Greig is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M. Beksac, Department of Hematology, Faculty of Medicine, Ankara University, Ankara, Turkey; S. Jagannath, The Tisch Cancer Institute, Mount Sinai Hospital, New York, NY, USA; E. M. Ocio, Complejo Asistencial Universitario de Salamanca (IBSAL), Centro Investigación del Cáncer-IBMCC (USAL-CSIC), Salamanca, Spain; A Palumbo, Division of Haematology, University of Torino, Torino, Italy; G. Pratt, School of Cancer Sciences, University of Birmingham, Heart of England NHS Foundation Trust, Birmingham, UK.
Rights and permissions
About this article
Cite this article
Greig, S.L. Panobinostat: A Review in Relapsed or Refractory Multiple Myeloma. Targ Oncol 11, 107–114 (2016). https://doi.org/10.1007/s11523-015-0413-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0413-6