Abstract
Purpose
The present study aimed to explore the glymphatic pathway in the intraorbital optic nerve (ON) using magnetic resonance imaging (MRI).
Methods
Following conventional MRI examination, a total of 89 outpatients underwent T2-weighted imaging in thin-sliced coronal and sagittal sections. Moreover, three injected cadaver heads were dissected.
Results
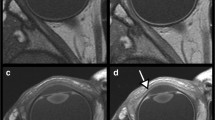
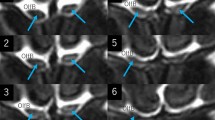
In the cadaver specimens, differences in appearance between the central and peripheral parts of the ON were not observed. On the axial T2-weighted MRI performed in the initial examination, the central part of the intraorbital ONs was delineated as a well-demarcated, linear hyperintense area in 19% of patients. On the thin-sliced serial coronal images, the hyperintense areas were identified on both sides in 91% of patients. They were delineated as continuous hyperintense areas in the ONs with an inconsistent appearance even in the same nerve. In 12.4% of patients, the areas were divided into the upper and lower parts by a horizontal septum, while others showed variable morphologies, lacking a septum. On thin-sliced sagittal images, hyperintense areas were identified in 46% of patients.
Conclusion
Hyperintense areas in the intraorbital ON detected on T2-weighted sequences may involve a glymphatic pathway with perivascular spaces of the ON and central retinal artery. These may be collapsed and difficult to identify on surgical and cadaver specimens.





Similar content being viewed by others
References
Baneke AJ, Aubry J, Viswanathan AC, Plant GT (2020) The role of intracranial pressure in glaucoma and therapeutic implications. Eye (Lond) 34:178–191
Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR (2008) Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci 49:5412–5418
Furlanetto RL, Teixeira SH, Gracitelli CRB, Lottenberg CL, Emori F, Michelan M, Amaro E Jr, Paranhos A Jr (2018) Structural and functional analyses of the optic nerve and lateral geniculate nucleus in glaucoma. PLoS ONE 13:e0194038
Garaci FG, Bolacchi F, Cerulli A, Melis M, Spanò A, Cedrone C, Floris R, Simonetti G, Nucci C (2009) Optic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 252:496–501
Hablitz LM, Plá V, Gianetto M, Vinitsky H, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M (2020) Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 11:4411
Hao J, Pircher A, Miller NR, Hsieh J, Remonda L, Killer HE (2020) Cerebrospinal fluid and optic nerve sheath compartment syndrome: a common pathophysiological mechanism in five different cases? Clin Exp Ophthalmol 48:212–219
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Godman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111
Jacobsen HH, Ringstad G, Jørstad ØK, Moe MC, Sandell T, Eide PK (2019) The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci 60:2773–2780
Killer HE, Laeng HR, Flammer J, Groscurth P (2003) Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol 87:777–781
Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH (2017) Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci 58:4784–4791
Mathieu E, Gupta N, Paczka-Giorgi LA, Zhou X, Ahari A, Lani R, Hanna J, Yücel YH (2018) Reduced cerebrospinal fluid inflow to the optic nerve in glaucoma. Invest Ophthalmol Vis Sci 59:5876–5884
Mestre H, Mori Y, Nedergaard M (2020) The brain’s glymphatic system: current controversies. Trends Neurosci 43:458–466
Mirra S, Marfany G, Garcia-Fernàndez J (2020) Under pressure: Cerebrospinal fluid contribution to the physiological homeostasis of the eye. Semin Cell Dev Biol 102:40–47
Papp A, Tóth J, Kerényi T, Jäckel M, Süveges I (2004) Silicone oil in the subarachnoidal space – a possible route to the brain? Pathol Res Pract 200:347–352
Price DA, Harris A, Siesky B, Mathew S (2020) The influence of translaminar pressure gradient and intracranial pressure in glaucoma: a review. J Glaucoma 29:141–146
Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, Wang H, Li B, Zhang X, Wang N (2010) Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology 117:259–266
Sakamoto M, Nakamura K, Shibata M, Yokoyama K, Matsui M, Ikeda T (2010) Magnetic resonance imaging findings of Terson’s syndrome suggesting a possible vitreous hemorrhage mechanism. Jpn J Ophthalmol 54:135–139
Sartoretti T, Stürmer J, Sartoretti E, Najafi A, Schwenk Á, Wyss M, Binkert C, Sartoretti-Schefer S (2019) Long segment 3D double inversion recovery (DIR) hypersignal on MRI in glaucomatous optic neuropathy. BMC Ophthalmol 19:258
Wostyn P, Van Dam D, Audenaert K, Killer HE, De Deyn PP, De Groot V (2015) A new glaucoma hypothesis: a role of glymphatic system dysfunction. Fluids Barriers CNS 12:16
Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP, Killer HE (2016) The glymphatic system: a new player in ocular diseases? Invest Ophthalmol Vis Sci 57:5426–5427
Wostyn P, Killer HE, De Deyn PP (2017) Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin Exp Ophthalmol 45:539–547
Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP (2017) The glymphatic hypothesis of glaucoma: a unifying concept incorporating vascular, biomechanical, and biochemical aspects of the disease. Biomed Res Int 2017:5123148
Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP, Killer HE (2018) The first histologic evidence of a paravascular pathway within the optic nerve. Invest Ophthalmol Vis Sci 59:1717
Wostyn P (2019) Glaucoma as a dangerous interplay between ocular fluid and cerebrospinal fluid. Med Hypotheses 127:97–99
Wostyn P, Mader TH, Gibson CR, Killer HE (2020) The perivascular space of the central retinal artery as a potential major cerebrospinal fluid inflow route: Implications for optic disc edema in astronauts. Eye (Lond) 34:779–780
Yiannakas MC, Toosy AT, Raftopoulos RE, Kapoor R, Miller DH, Wheeler-Kingshott CA (2013) MRI acquisition and analysis protocol for in vivo intraorbital optic nerve segmentation at 3T. Invest Ophthalmol Vis Sci 54:4235–4240
Zhang YQ, Li J, Xu L, Zhang L, Wang ZC, Yang H, Chen CX, Wu XS, Jonas JB (2012) Anterior visual pathway assessment by magnetic resonance imaging in normal-pressure glaucoma. Acta Ophthalmol 90:e295–e302
Acknowledgements
This study did not receive any grant funding.
Author information
Authors and Affiliations
Contributions
ST and HO developed the study project. ST performed cadaver dissection. HO performed examinations using magnetic resonance imaging. HI collected and managed the data. ST and HI analyzed the data. ST wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest concerning the materials and methods used in this study or the findings presented in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsumi, S., Ono, H. & Ishii, H. Hyperintense areas in the intraorbital optic nerve evaluated by T2-weighted magnetic resonance imaging: a glymphatic pathway?. Surg Radiol Anat 43, 1273–1278 (2021). https://doi.org/10.1007/s00276-020-02649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-020-02649-7