Abstract
Wetlands, namely the riparian ones, play a major role in landscape and water resources functionalities and provide enormous opportunities for ecosystems services. However, their area at globe scale is continuously decreasing due to appropriation by the riverain communities or by allocation of water resources to other uses, namely irrigation, in prejudice of natural wetlands. Due to the high competition for water, namely for agricultural irrigation, the calculation of the vegetation evapotranspiration (ETc), i.e. the consumptive water use of the wetland ecosystems, is mandatory for determining water supply–demand balance at various scales. Providing for the basin and local levels the reason for this review study on ETc to be presented in an irrigation focused Journal. The review also aims to make available adequate Kc values relative to these ecosystems in an ongoing update of FAO guidelines on evapotranspiration. The review on ETc of natural wetlands focused on its computation adopting the classical FAO method, thus the product of the FAO-PM grass reference ETo by the vegetation specific Kc, i.e., ETc = Kc ETo. This approach is not only the most common in agriculture but is also well used in natural wetlands studies, with Kc values fully related with vegetation ecosystems characteristics. A distinction was made between riparian and non-riparian wetland ecosystems due to differences between main types of water sources and main vegetation types. The Kc values are tabulated through grouping wetlands according to the climate since the variability of Kc with vegetation, soil, and water availability would require data not commonly available from the selected studies. Tabulated values appear to be coherent and appropriate to support field estimation of Kc and ETc for use in wetlands water balance when not measured but weather data may be available to compute the grass reference ETo. ETc and the water balance could then be estimated since they are definitely required to further characterization and monitoring of wetlands, defining measures for their protection, and assessing ecosystems’ services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of wetlands in influencing water resources, giving relevance to biodiversity and ecosystems services, and in terms of climate change has been widely recognized (Mitsch and Gosselink 2015; Mitsch et al. 2015). Nevertheless, worldwide, the loss of every type of wetlands is continuing (Dixon et al. 2016; Hu et al. 2017). Wetlands are ecosystems of high ecological value and the most biologically rich on the planet (Hauenstein et al. 2014). Each wetland differs due to variations in soils, landscape, climate, water regime and chemistry, vegetation, and human disturbance. This variability of characteristics is well evident through the performed review, resulting that vegetation and ecosystem evapotranspiration varies enormously with those characteristics.
Under the Ramsar Convention (UNESCO 1994) wetlands are areas of marsh, fen, peatland or water presence, whether natural or artificial, permanent or temporary, static or flowing, fresh, brackish or salty, including areas of marine water where its depth at low tide does not exceed six metres. Various classifications of wetlands are in use (EPA 2001; Hu et al. 2017; Ilhardt et al. 2000; Mitsch and Gosselink 2015; Zaimes et al. 2007). Because of their high conservation value, many habitats found in wetlands have been included in the European Habitat Directive (92/43/EEC) (Council Directive 1992), while many of them have been denominated as Nature Reserves, or are Parks, Special Conservation Areas, or other national protected areas. Where river mouths meet the sea, occur unique wetland complexes such as marine and coastal wetlands. The vegetation in these areas ranges from riparian habitats, which differ little from habitats associated with the river upstream, to salt marshes that have a distribution limited to tidally influenced areas with high water salinity. The processes that drive the distribution and dynamics of plant communities in river estuary wetland complexes are also varied, ranging from fluvial-dominated processes, such as flooding and channel migration, to marine processes such as tidally driven water-level fluctuations (Shafroth et al. 2020). Few species can develop and reproduce with repeated exposure to sea water, the halophytes. The latter play an increasingly important role as models for understanding the salt tolerance of plants, or as genetic resources that contribute towards the improvement of salt tolerance in some crops, or for the re-vegetation of saline lands, and as ‘niche crops’ in landscapes with saline soils (Flowers and Colmer 2015). These coastal systems are among the most vulnerable and threatened habitats on a global scale (Sarika and Zikos 2020).
In inland wetlands, aquatic vegetation typically grows as monospecific patches within streams with patterning caused by self-organization processes. The presence of submerged plants in ponds depends on the season and the water level. The larger aquatic plants that grow in wetlands are the macrophytes, which use solar energy to produce organic matter, which subsequently provides the energy source for heterotrophs (animals, bacteria and fungi). The water and nutrient supply in wetlands make that the primary productivity of ecosystems dominated by wetland plants are among the highest in the world (Adame et al. 2017; Hirota et al. 2007; Miller and Fujii 2010). A high heterotrophic activity is usually associated that creates a high capacity to decompose and transform organic matters and other substances. Macrophytes may be classified as:
-
i.
Emergent: These are the dominating life form in wetlands. Examples include the common reed, palmiet, sawgrass, cattails, bulrushes, watergrass, which often form the border of the wetland or the transition to the upland.
-
ii.
Floating-leaved: These includes species that are rooted in the substrate, e.g. waterlilies, and species that float freely on the surface of the water, e.g. water hyacinth. The freely floating species are highly diverse in form and habit.
-
iii.
Submerged: These have their photosynthetic tissue entirely submerged while the flowers are usually exposed to the atmosphere.
The many functions of wetlands include contributing to the life cycle of plants and animals, by providing habitat, food, nesting sites and shelter for many wildlife species (Nayak and Bhushan 2022). They also moderate climate changes, act as CO2 sinks, dampen the effect of waves and store floodwaters, retain sediment, and reduce sedimentation and pollution. They are important in the production of food and fodder for domestic and wild animals, and are a source of raw materials for handicrafts (Xu et al. 2020). These animals include alligators, cicadas, manatees, caimans, bullfrogs, beetles, crustaceans, and beavers. Each of these creatures has adapted to life in the wetland environment and plays an important role in keeping the health of the ecosystem. Animals include very rare birds, e.g., the Madagascar pochard (Aythya innotata), whose estimated population is less than 150, as analysed by Rose (2021) for birds.
The riparian wetland zone is usually defined as part of the landscape that borders a body of water. These water bodies can be natural, such as streams, rivers and lakes, or man-made, such as ditches, canals, ponds and dams (Zaimes et al. 2007). The active riparian zone refers to a saturated/unsaturated region that is hydrographically connected to the watercourse and directly influences the flow (Sarwar et al. 2022). Verry et al. (2004) proposed and discussed the definition of riparian ecotone as an interaction space comprising terrestrial and aquatic ecosystems. Riparian ecosystems include the physical environment and biological communities that lie at the boundary between freshwater and terrestrial systems. The riparian zone consists of a bank at the channel edge and various types of landforms adjacent to the bank, formed mainly by colluvium deposits in mountainous regions and alluvial deposits in other contexts, with a larger valley floor and a visible floodplain (Dufour et al. 2019). Riparian wetlands can be associated with perennial, intermittent and ephemeral rivers. Perennial streams have in-channel flows all year round and have substantial groundwater flows. Stream flows can vary widely from year to year and may even dry up during severe droughts, but the groundwater table is always near the surface. Perennial streams are found in both humid and arid regions (Zaimes et al. 2007). When the streams are intermittent or ephemeral, they become under a torrential regime, trees may disappear, and shrubby vegetation grows on the banks. Zaimes et al. (2007) referred that intermittent streams/riffs are also linked to the groundwater, which is located immediately below the stream bed, even if there is no flow. These streams are usual in arid and semi-arid climates, with the oasis belonging to this group of riparian vegetation. The riparian ecosystems are the most dynamic, productive, and vulnerable ecosystems in drylands, and they are centres of human life and economic development in arid and semi-arid regions (Chen et al. 2023); thus, because water is the scarcest and most constrained resource in drylands, increasing water demand could potentially threaten regional water security. The Ejina oasis, in NW China, located at the interface between Alasan highlands and desert is an example (Hou et al. 2010). In such arid regions, water resources become the key driving factor of ecological environment evolution, and it is the main constrain to the maintenance of ecosystems. The riparian zones are transition zones and have characteristics of both aquatic and upland terrestrial ecosystems (Zeng et al. 2020). This is reflected in the presence of a greater number and diversity of species (Zaimes et al. 2007). The characterization of wetlands ecosystems needs to include the evapotranspiration (ETc), i.e., the consumptive water use of the ecosystem, and the water balance, which determine the dynamics of the relationship between supply and demand. Their knowledge allows to better define how to protect the system and assessing the ecosystem services.
Riparian wetland ecosystems are recognised as highly diverse and containing specialised ecological communities as well as providers of multiple ecosystem services (Kaletová et al. 2019; Xu et al. 2019; Mandžukovski et al. 2021). The most important functions of riparian vegetation (Zaimes et al. 2007; Dickard et al. 2015; Zeng et al. 2020) are: to enhance habitat for terrestrial and aquatic fauna; to retain sediments and nutrients resulting from runoff or from flooding; to mitigate the pollution resulting from agriculture through detention, buffering and processing; to stabilise and to strengthen the stream banks; to store water and recharge the underground aquifers; to reduce flood runoff. The functional riparian zones enhance the water quality and carbon sequestration (Mandžukovski et al. 2021). Well-functioning riparian zones improve regional biodiversity, and the resilience of landscapes to climate change and related hydro-climate impacts (Mandžukovski et al. 2021). Better knowing these functions require knowledge of the dynamics of ETc and of the wetland water balance. Biological importance is also linked to habitat availability and the role of wildlife corridor (de la Fuente et al. 2018), and the vegetation's effect on biodiversity. On a social level (Dufour et al. 2019), riparian vegetation contributes to the landscape identity to which it is a part; for example, providing a contribution to creative services (recreation, inspiration, etc.). Climate has a strong influence on the function and structure of the riparian zones through temperature, precipitation, evapotranspiration, and runoff (Verones et al. 2013), which determine their water balance and recognizing the role of flood events as influencing the species composition.
The agriculture zones are usually located on river floodplains, and use water diverted from the river to irrigate crops. Agriculture is the primary source of income for the surrounding communities; thus resource managers need accurate estimates of water requirements of both crops and riparian vegetation and allocate water. Water governance must have that knowledge and the voice of the riverain communities; otherwise, wetlands decline as analysed by Nouri et al. (2023) for Iran where water is increasingly used for irrigation in arid zones. The major direct change in converting a riparian area to agricultural area is the change in vegetation and consequently in ETc, i.e., the consumptive water use, and area water balance or alteration of the supply–demand dynamics. Various studies have identified negative (Galbraith et al. 2005) and positive (Sueltenfuss et al. 2013) interactions between wetlands and irrigation.
The increase in cropped area can lead to higher peak flows and subsequent flooding downstream. Increased flows in the stream channel in turn lead to greater stream incision and stream bank erosion. The base flow decreases because water flows out of the watershed very fast. These changes alter the local hydrologic cycle and have converted many perennial streams into intermittent or ephemeral streams (Zaimes et al. 2007). For example, in the middle reaches of the Tarim River (China), cropland expanded with increasing population and demand for grain. As a result, increasing amounts of runoff from the upper reaches were captured and used to irrigate crops. This has resulted in less runoff entering the lower reaches of the river, causing there a variety of problems such as river-flow interruptions, drying up of terminal lakes, shrinkage of natural vegetation areas, land desertification, intensified sandstorms, soil salinization, and declining water quality (Mamat et al. 2018). This example also evidences the need for knowing ETc and the water balance if the protection of the riparian vegetation is intended, namely to balance the potentially negative impacts of agriculture. Riparian zones may play a crucial role in the water balance of a catchment and in the hydrological process (Yu et al. 2016; Sarwar et al. 2022).
To maintain the biodiversity of riparian and wetland vegetation around the world, it is important to recognize that these zones are vital areas of interaction between land and water bodies and are often degraded by various pressures such intensive agriculture and river engineering works (Urbanič et al. 2022). The most severe and common human impacts are due to land-use conversion to agriculture, streamflow regulation, nutrient enrichment, and climate change. Adopting an integrated socio-economic and environmental dynamic perspective will ensure the sustainable management of riparian and wetlands. In light of climate change, it is critically important to conserve and/or restore the ecological integrity of these areas. Plant species in all regions are adapted to multiple abiotic stressors, including dynamic flooding and sediment regimes and seasonal water shortage. Current knowledge gaps and subjects for future research include cumulative impacts to small, ephemeral streams and large, regulated rivers, as well as understudied ecosystems in North Africa, the western Mediterranean basin, and Chile (Stella et al. 2013). Research on vegetation evapotranspiration is helpful in understanding the water balance of riparian forests, especially in the extreme arid regions, and can be used to determine the actual ecological water demand of desert riparian forest and other areas. Thus, research can contribute to the rational use of water resources, protection, and maintenance of the stability of the riparian forest system and wetland vegetation (Hou et al. 2010). An example of native plants reestablishment by controlling invasive saltcedar (Tamarix spp.) in riparian areas of the Southwestern USA by inducing its transpiration reduction is reported by Solis et al. (2024). Updated knowledge on management of riparian wetlands is provided by Johnson et al. (2018) and Carothers et al. (2020).
In order to study, monitor, design and implement protection and conservation measures, it is necessary to determine the water balance of wetland systems and therefore to have a reliable and simple methodology for computing evapotranspiration. The referred FAO approach – ETc = Kc ETo – is already in use in agriculture and in multiple natural on-site studies. Its simplicity and the fact that Kc represents the different responses of the vegetation and varies with the location, particularly with the wetness or dryness of the climate and the soil and vegetation. When recognizing the seasonal ETc characteristics of the ecosystems, the characteristics of climate and the typical vegetation, it is possible to transfer in space the Kc values and provide for a simple estimation of ETc of the vegetation in a different location. This procedure is in use after long time, reason why updates for tree and grass crops were recently published (Pereira et al. 2023a, b), so preceding this paper. Naturally, the precision of estimators may not be very high because wetlands are complex systems and it depends on the knowledge and experience of researchers, but it may be quite precise in terms of experimental approach. As a starting point for the application of this approach, there is the need for a wide estimation of the Kc values.
The main objective of the study therefore consists of the review collection of available Kc values for a variety of wetlands and riparian ecosystems, which is resolved through the tabulation of Kc and the related basic characteristics of respective locations. An essential objective is the use of tabulated Kc with ETo computed from local observations to easily and accurately estimate ETc, so to calculate the areal water balance and the supply demand balance focused on the protection of wetlands and riparian zones and on the assessment of their ecosystem services. Another objective consists of using the tabulated Kc to select a set of wetlands and riparian ecosystems to update the wetlands Kc section in the revised version of FAO56 guidelines (Allen et al. 1998). A further objective is to make available a tabulated collection of Kc values that can be used to check field results in the research practice. An additional objective is to make available such tabulated Kc values for use in water resources studies where water balance and demand–supply studies are required.
Material and methods
Evapotranspiration
The current review based upon collecting published evapotranspiration studies that reported on crop coefficients (Kc) for natural wetlands and riparian vegetation ecosystems, providing for easy calculating ecosystem (ETc) with the FAO procedure (FAO56, Allen et al. 1998). As introduced earlier, this method uses the product of the grass reference ETo, defined with the equation FAO-PM ETo (FAO56, Allen et al. 1998), by a crop (vegetation) coefficient Kc that represents the differences between the considered crop (vegetation) and the grass reference crop in terms of transpiration by the canopies and soil evaporation. Thus, Kc is defined by the ratio ETc/ETo. The review was performed through the widest possible internet search focused upon papers reporting on Kc obtained from field measurements of actual ETc of wetlands and riparian vegetation (ETc act).
The daily rate of evapotranspiration ET [mm d−1] can be computed with the Penman–Monteith combination equation (PM, Monteith 1965):
where λ is the latent heat of vaporization [kg m−3], Rn-G is the net balance of energy available at the surface [MJ m−2 d−1] computed as the difference between the net radiation Rn and the soil heat flux G, (es—ea), difference between the vapor pressure of the air at saturation es and at actual conditions ea, represents the vapor pressure deficit (VPD) of air at the reference (weather measurement) height [kPa], represents mean air density [kg m−3], cp represents specific heat of air at constant pressure [kJ kg−1 ºC−1], is the slope of the saturation vapor pressure–temperature relationship at mean air temperature [kPa ºC−1], is the psychometric constant [kPa ºC−1], rs is the (bulk) surface resistance [s m−1], and ra is the aerodynamic resistance [s m−1]. Equation 1 is used to compute ETc act from field observations; it would be often used in practice if the resistances rs and ra of the considered vegetation would be easily known. Several studies report that these parameters have to be calibrated using field measurements of ETc act with eddy covariance (EC) systems, or the Bowen ratio energy balance (BREB) instrumentation, as referred to in the tables hereafter. However, rs and ra cannot but exceptionally be standardized, resulting that the PM Eq. (1) is not commonly used predictively.
The PM Eq. (1) was used to develop the FAO-PM ETo equation for the grass reference crop, which was standardized as a hypothetical crop with an assumed height of 0.12 m having a fixed daily average surface resistance of 70 s m−1 and an albedo of 0.23, closely resembling an extensive surface of green grass of uniform height, actively growing, free of diseases and adequately watered. This definition allows the parameterization of rs and ra and, replacing them in Eq. 1, in the FAO-PM ETo equation, thus (Allen et al. 1998, 2006):
where, in addition to the variables defined for Eq. (1), T is mean daily air temperature [°C] and u2 is wind speed [m s−1], both measured at 2 m height. For daily computations, the surface resistance is assumed rs = 70 s m−1, resulting Cn = 900 and Cd = 0.34. When using an hourly time-step computation Cn = 37, while Cd = 0.24 during daytime (Rn > 0), assuming rs = 50 s m−1, and Cd = 0.96 during nighttime because rs = 200 s m−1.
The American Society of Civil Engineers (ASCE) adopted both the grass and alfalfa reference, respectively ASCE-PM ETo and ASCE-PM ETr, with alfalfa having a standardized height of 50 cm, then having different values for Cn and Cd (Eq. 2). Due to the lower rs and higher ra of alfalfa, ETr ≈ 1.15 ETo. This ratio is adopted in the current study.
The computation of the PM-ETo equation parameters should follow the procedures described by Allen et al. (1998) but, in many parts of the world, data on some weather variables are often missing, or are of low or questionable quality, namely solar radiation, air humidity and wind speed. The missing variables can then be estimated using the procedures proposed by Paredes et al. (2020, 2021) and in the new coming FAO56 revised, namely referring to the Hargreaves equation (HS), or to the use of reanalysis weather data or stationary satellite grided data (Allen et al. 2021; Paredes et al. 2021).
Crop coefficients
Assuming the FAO approach to compute ETc
it is important to recall that, being solely computed with weather variables, not observed, ETo represents the climatic demand on evaporation. Kc represents an integration of the effects of three primary characteristics that distinguish the crop or vegetation from the reference: the crop height, h, and the aerodynamic resistance; the crop-soil surface resistance, rs and the albedo, α, of the crop-soil surface. Thus, Kc is little influenced by the climate and standard Kc values for the same crop or type of vegetation can be transferred between locations and climates.
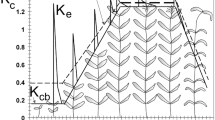
In the current study, a time-averaged Kc is adopted since it includes multi-day effects of evaporation and transpiration. For wetlands and riparian vegetation these effects are generally combined and there is no need to separate evaporation and transpiration. The changes in Kc over the growing season are represented by the crop coefficient curve (Fig. 1), which relates to changes in the vegetation and ground cover through the crop season that affect the ratio ETc/ETo at the four growth stages considered: initial, development, mid-season and late season as per Fig. 1.
Typical crop coefficient curve referring to four growth stages and the main factors affecting their duration and variation with the type of vegetation and crop management (source: Allen et al. 1998)
The Kc generally increases from an initial value, Kc ini, until reaching a maximum, Kc mid, at the mid-season period, the time of maximum or near maximum plant development (Fig. 1). During the late season period, vegetation progressively senesces or becomes dormant, and leaves senesce and eventually dry out and fall, the Kc generally decreases until it reaches a lower value, Kc end, at the end of the plant season. The late season ends quickly, sometimes abruptly, when frost affects the vegetation and induces their dormancy, i.e., the length of the late-season period may be relatively short for vegetation killed by frost. The length of plant stages is vegetation-specific and change duration with weather conditions, mainly air temperature. The lengths of the initial and development periods may be relatively short for deciduous trees and shrubs that develop new leaves in the spring at relatively fast rates. The value for Kc end should reflect the canopy condition of the vegetation immediately before plant death.
The four crop growth periods in Fig. 1 are: (i) Initial: for annuals, the duration of this phase is from planting date or, for perennials, from the "greenup" date when initiation of new leaves occurs, to approximately 10% ground cover; (ii) Development: from 10% ground cover to effective full cover; (iii) Mid-Season: from effective cover to start of maturity or senescence, often indicated by the beginning of the ageing, yellowing or senescence of leaves or leaf drop; (iv) Late Season: from start of maturity or senescence to full senescence or dormancy. To draw the Kc curve three Kc values have to be known—Kc ini, Kc mid and Kc end -, which need to be connected by straight line segments through each of the four growth stage periods. This procedure applies to all types of vegetation but with different durations of crop stages (Fig. 2), which must be identified by the users.
Crop growth stages for different types of vegetation, annuals and perennials, deciduous and evergreen, compared with the grass reference crop (source: Allen et al. 1998)
Annual crops tend to have longer initial, development and late-season durations than perennials. Among the latter, grasses, reeds and deciduous trees and shrubs may have short initial and development stages depending on climate. Differently, evergreen trees and shrubs may do not have a distinction among phases, depending upon climate. This variability is evident in Tables presented in “Crop coefficients for wetlands” and “Crop coefficients for wetland riparian ecosystems” sections for both wetland and riparian ecosystems. Vegetation Kc varies throughout the growth season with variations in physiologic plant response to the atmosphere demand, but Kc is time averaged for the initial and mid-season stages referred above, thus are then considered not changing. For research purposes, it may be of interest to consider it variable, e.g., depending upon the indices of vegetation (VI) as defined in remote sensing. Glenn et al. (2011) proposed the use of Kc estimated as a function of the variable parameter EVI. However, considering the variability of mixed vegetation, the time averaged Kc is adequate for most uses. In remote sensing, Kc values are often replaced by the index EToF, fraction of ETo, which varies with time (Glenn et al. 2011).
Field research methods in wetlands literature included: (i) the soil water balance (SWB) based on observations of the soil water content using soil sampling or various types of sensors; (ii) the catchment hydrologic water balance (HWB); (iii) the Bowen ratio energy balance (BREB); (iv) the eddy covariance system (EC); (v) weighing, drainage and water table lysimeters (WL, DL and WTL); (vi) mini or micro lysimeters (ML) to assess soil evaporation; and (vii) diverse but consistent empirical methods such as testing different Kc values against observed yields. Most field methods are analyzed for accuracy by Allen et al. (2011).
The methods used to compute and assess ETc act, in addition to the FAO56 method (Allen et al. 1998), included the Penman method (Doorenbos and Pruitt 1977), the Penman–Monteith combination equation (Monteith 1965, PM), the Priestley and Taylor equation (1972, PT), and the double source method of Shuttleworth and Wallace (1985, SW). The PM, the PT and the SW equations require specific field methods. Several studies were performed with support of models with proper calibration. The most used software models comprise SIMDualKc (Rosa et al. 2012) and HYDRUS (Šimůnek et al. 2016). In addition, remote sensing (RS) was largely used, mainly in the last decade, and surface energy balance models (SEB), e.g. METRIC, SEBAL and SEBS (Allen et al. 2007), and RS vegetation indices (RSVI, Pôças et al. 2020), were used. A list of symbols, acronyms and abbreviations is included at end of the article in Abbreviation section.
Selection and use of bibliography data
The search was performed in Science Direct and through the pages of various journals, as well as using bibliography listed in selected articles. English, Spanish, Portuguese, French, Italian and German languages were considered. The search keywords, in addition to evapotranspiration, crop coefficients, wetlands and riparian vegetation, included designations of common plants of these ecosystems. Only full articles were reviewed. Papers were selected when they evidenced good/satisfactory quality of field research, regardless of the journals in which they were published, and where both ETo and ETc were appropriately calculated/estimated. The approach described was adopted in previous Kc reviews by Pereira et al. (2023a, b). Generally, selected papers required:
-
1.
The use of the grass reference FAO-PM-ETo equation.
-
2.
That the conversion ratio from the used ETo or ETr equation to the FAO-PM-ETo, was known.; a conversion factor of 1.15 was used when the ASCE-PM ETr equation for alfalfa (Eq. 2) or the Penman equation were adopted.
-
3.
The field methods were well described and should refer to consistent methodologies that provide for computing ETc act accurately as proposed by Allen et al. (2011).
-
4.
The Kc values should have been derived from adequate field research and well discussed.
-
5.
Tthe Kc values were provided in Tables, graphics or in the text, preferably with a specific discussion; for a few cases, when only ETc act and ETo were provided, Kc (average) values were computed. Otherwise, data were not considered.
-
6.
The description of the studied wetland or riparian vegetation, with identification of main species, should be as complete as possible.
-
7.
The computation methods should be sufficiently descriptive, and models should be calibrated and validated in line with the recommendations by Allen et al. (2011) to understand if the reported methods provided for reliable data. Otherwise, the study was not considered.
Crop coefficients for wetlands
The search on wetlands actual ET (ETc act) and Kc provided a good number of case studies covering most of the wetland types, many of them recognized by the RAMSAR Convention. However, since not all authors classified the described wetlands, their analysis could not be performed according to a common wetland classification, nor in relation to the dominant vegetation due to its very wide variability. The option was then to group the reported wetland case studies according to the climate since it influences enormously the type of dominant vegetation and its evapotranspiration and Kc values. Related information was summarized in Tables, which are quite useful for readers to compare among the table-grouped case studies.
The Tables 1, 2, 3, 4 and 5 provide information on the location of the studied sites, the authors, the dominant vegetation species, the methods used for estimating of ETo and ETc act, which allow readers to better compare results and to perceive the confidence on their computation, and the actual crop coefficients derived from field observations for the initial stage, the mid-season and the end season, as well as the average value for the vegetation season. When opportune, conditions relative to the estimated Kc are also referred, e.g., the duration of the vegetation cycle. Differently, Table 6 provides summarized information about diverse vegetation species whose data could be duly assigned to them. Plants nomenclature, morphology and ecology in Table 6 are those referred in a flora site where the third author collaborates: http://www.worldfloraonline.org.
Table 1 presents Kc values relative to wetlands located in regions of freezing winter climates but warm summer in high elevation areas, thus where the vegetation season is short, about 6 months. Killing frost is likely to occur there, which could induce end season Kc to fall abruptly after very low temperatures would cause a sudden period of dormancy. In the case of meadows, which are quite common in these regions, Kc end may be high, close to Kc mid, due that abrupt transition to dormancy, or low if dormancy is not completely abrupt. The first condition likely occurred in the two meadows on the Qinghai–Tibet Plateau (Dai et al. 2021; Guo et al. 2022), while the Kc end values does reflect a slow entrance in dormancy in case of meadows reported by Yang et al. (2017) and Li and Wang (2015). Other wetlands, with more complex vegetation reported, may have been affected by abrupt high cold in the Fall, but the Kc end values do not reflect well that condition or are lacking (Baeumler et al. 2019; Hwang et al. 2020). Also likely influenced by freezing winter is the case of high elevation Andean “páramos” of Ecuador, reported by Carrillo-Rojas et al. (2019), which shall have high values of Kc ini and Kc end since the average Kc equals Kc mid.
The actual Kc values in the initial stage are extremely varied, likely influenced by the timing of field observations and by the response to the climate. When field research starts early in the season, the Kc ini value is low; contrarily, it is high when observations start late. However, the initial conditions could not be assessed when reviewing the papers. The case of mountain swamp meadows reported by Li and Wang (2015) and Yang et al. (2017) likely refer to vegetation that responded slowly to the atmosphere demand. Differently, the studies reported by Dai et al. (2021), Guo et al. (2022), Baeumler et al. (2019), Hwang et al. (2020) and Carrillo-Rojas et al. (2019) probably are cases where the initial stage was quite long, ending when evapotranspiration was close to ETc mid.
The Kc mid values and the season Kc avg vary less than the initial and end Kc values because both represent several weeks or month averages. The lowest Kc mid value refers to a high elevation Andean páramo of Ecuador (Buytaert et al. 2006), likely not used for grazing since it is referred that plant leaves fall and create a mulch that highly reduces ETc. Its results are not comparable with those reported for another high elevation Andean páramo that has a Kc value that double the former and is used for grazing (Carrillo-Rojas et al. 2019). Generally, meadows and grasslands have relatively high Kc mid values, from 0.85 to 1.26, and Kc avg often close to 1.0.
The wetlands in mostly temperate regions having cold winter and a warm summer are described in Table 2. These study cases mostly refer to Europe while those in more stringent climates refer to China and Turkmenistan. Reeds in swamps and marshes are the most common vegetation and have high Kc mid values, generally higher than 1.00, up to 1.50. This high value of 1.50 reported by Anda et al. (2014) is likely due to local advection contributing to the energy used for evaporation, which probably occurs if patches of high reed are surrounded by low vegetation. Reeds have high Kc mid, larger than or close to 1.0, and lower values of Kc ini and Kc end, thus with a Kc curve like the classical segments curve proposed by FAO (Doorenbos and Pruitt 1977). Grasses show a Kc avg close to 1.0 and a behavior like that of the reference grass. Diverse vegetation occurs by the Fenéka pond (Anda et al. 2015) to which correspond varied Kc avg values. The highest Kc avg values there, of 1.18, refer to emergent vegetation, mainly reed and cattail, and the smaller Kc avg values are of woody deciduous trees and shrubs, 0.75 and 0.85. Coastal wetland plants also show high Kc mid (Fermor et al. 2001; Jia et al. 2009). Information collected indicates a large daily variation of Kc values, mainly depending on the available solar energy, i.e., ETc of non-riparian wetlands is not water limited but energy limited.
Table 3 groups the selected case studies relative to sites with less-cold winter climate and with relatively high ET during the non-growing season. Most cases refer to Florida and California while the other cases are for diverse countries, regions. The dominant vegetation is varied, with cattail being the most occurrent in Florida and San Joaquin Delta, while reed is most common in Turkey, Germany, Italy, and China, where the temperature difference between summer and winter is notably higher. Cattail and reed Kc mid values, approximately 0.80 to 1.20, are nearly similar to those grown in cold and freezing winters (Tables 1 and 2) likely because summer weather environment is warm/hot for all three cases. Average Kc values for the growing season are generally smaller than Kc mid for the same period. Naturally, Kc avg for the growing season are notably higher than Kc avg relative to the non-growing season.
The values for the initial and end stages of reed and cattail are evidently smaller than Kc mid but may be close to that value. Likely this occurs when climate favours ET and a rapid initiation and/or energy keeps available during senescence. Generally, they are not very different of those reported for cold winter climates, likely because respective climate conditions during the growing season are somewhat similar. Cladium jamaicense and Cladium mariscus, reported respectively by Mao et al. (2002) and Wu and Shukla (2014), have shown a Kc behaviour similar to cattail but larger Kc. The tule (or bulrush), reported by Howes et al. (2015) for the Kern River in California, has also similar Kc values. The invasive Spartina alterniflora has shown to dominate over the common reed, growing faster and more and having higher Kc values than reed.
Table 3 includes various associations of diverse plants. The one reported by Min et al. (2010) is quite varied resulting in medium values for Kc relative to the three crop stages, which is proxy of values for cattail in various sites. Moffett et al. (2010) reported about another association of plants adapted to salinity in an intertidal salt marsh, in San Francisco Bay, California, USA. The ecosystem Kc avg is around 1.0, thus indicating a high response to the evaporative demand of the atmosphere. Another salt marsh in Newcastle, Australia (Hughes et al. 2001), with different vegetation, shows smaller Kc avg values.
Table 3 includes reference to an experiment for evaluation of various hydrophyte plants that have potential to be used as bioenergy crops (Barco et al. 2018). Results do not characterise any wetland because correspond only to single species; Kc values are high because the experimental sites were small and could be affected by local advection. However, these data (Barco et al. 2018) is important to recognize the ET demand of these plants.
Crop coefficients for wetlands in warm climates, that are often marked by aridity, including for wetlands near the sea and affected by salinity, are presented in Table 4. Vegetation tolerant to salts tends to dominate in coastal areas, while inland marshes also have salt tolerant plants. Kc values tend to be smaller in areas marked by salinity, but Kc mid values > 0.50. However, higher values are achieved when the vegetation is salt tolerant, while elsewhere most plants show a Kc mid value of 1.00 to 1.20 approximately, and Kc avg slightly smaller. Reported Kc values indicate higher values for small stands due to local advection.
The selected case studies in Table 4 refer to both arid and semi-humid climates, such as the swamps of the upper Nile and coastal wetlands of Maputaland, South Africa. Relative to the enormous wet savanna of Pantanal in Central Brazil, Kc avg data refers only to a forest. Relative to the vegetated drainage channels in Northern Egypt, Kc avg is reported for diverse species ranging 0.79 to 1.05. Despite the variability of vegetation, cattail, reed and tule are present in various sites with Kc mid values generally > 0.90.
Table 5 refers to peat bogs of varied origins and with the presence of moss, with the exception of a high mountain peatland area near the source of the Yangtse, in West China, where the dominant plants are Carex and Kobresia grasses. The case studies refer to different countries and environments, most cases in mountain sites, including in an island of Azores, and one case study in lowland, in the Netherlands. Apart from moss, the dominant vegetation is very diverse. Molinia caerulea is the most common plant in North Europe bogs, contributing to ecosystem Kc mid > 1.0, while the site of Santa Bárbara Mountain, Azores, has a Kc mid = 0.80 dictated by Calluna vulgaris. This lower value is likely due to the effects of high cloudiness limiting the energy available for evapotranspiration.
Table 6 presents typical Kc values and characteristics of vegetation recorded from the selected papers quoted in Tables 1, 2, 3, 4 and 5. The listed plants include only those that could be individuated through the papers, mainly in terms of Kc values and characteristics influencing those values. The Kc values tabulated are assimilated to standard values, thus generally assumed not stressed; smaller values are likely to be found for similar vegetation. The tabulated aspects characterizing the plants have various sources following a wide internet search, mainly http://www.worldfloraonline.org.
While data on Kc in Tables 1, 2, 3, 4 and 5 aims at supporting selection of Kc values by users in relation to the climate, vegetation and type of reported wetlands, Kc values in Table 6, in addition to be also used in the practice, aim at better characterizing vegetation dominating in the various reported wetlands. This type of characterization could be continued in future. The Kc mid values of emergent plants are generally in the range of 1.10 to 1.20 (Table 6) while small stands may have values 0.20–0.25 greater than large stands due to local advection. For common reed a difference in Kc values of about 0.10 between growing in standing water or in moist soil was detected. Lower Kc values are defined for emergent plants in salty wetlands, such as coastal marshes, with the smallest value for salt grass in intertidal marshes. Low values are also assigned to submerged or floating plants in ponds. Emergent grasses in salty marshes have Kc slightly lower than in meadows. These derived Kc values may be usable for establishing practical soil water or basin balances to estimate wetland evapotranspiration, perform the water balance and estimate the water requirements for conservation of the ecosystem, as well as to help detecting water supply deficits.
Crop coefficients for wetland riparian ecosystems
Riparian ecosystems occur in less widespread climates and environments than wetlands, being more frequent in temperate and warm climates marked by aridity. Their vegetation, contrarily to that of wetlands, does not require to live in standing water or in moist soil, but uses the close-by rivers’ water or a variably deep flowing groundwater. Therefore, riparian vegetation is quite different from that in wetlands, mainly consisting of trees (e.g., cottonwood and willow) and large or small shrubs (e.g., tamarisk and mesquite), namely having deep roots. Plants nomenclature, morphology and ecology are those referred in http://www.worldfloraonline.org. Differently from wetlands, the available water may vary much from year to year and season to season depending upon the variations in precipitation, basin runoff (RO) and water infiltration and recharge of groundwater (GW), as well as with water withdraws from the river or groundwater for human and societal uses, typically for irrigation.
Table 7 presents the Kc values and information from papers relative to riparian vegetation ecosystems occurring in regions of cold winter climates and warm to hot summer. Most studies refer to the arid/semiarid regions of northwest China and south/southwest of USA. Other studies refer to UK, Denmark, Hungary, and Turkmenistan. That diversity of studies and sites shall provide a good vision of riparian ecosystems in the North Hemisphere. The dominant vegetation in NW China consists of tamarisk and saxaul, two large shrubs, and cottonwood and Mongolica pine as main trees, but tamarisk and cottonwood are by far dominating. Both tamarisk and cottonwood are also dominating in north America, together with other shrubs and trees, such as the Russian olive, a large shrub, and willow and juniper trees. The Kc mid values of tamarisk and cottonwood in Chinese sites are generally less than 1.0 but when water is not limiting are greater, 1.23 in case of Populus gansuensis. Other trees and shrubs commonly have values of Kc mid < 0.70, i.e., riparian plants mostly are water limited.
Typical riparian ecosystems using groundwater tables are those in an oasis and along the Heihe and Tarim rivers. having lower Kc values when salinity occurs and in downstream areas due to high water withdraw upstream. To note that Apocynum pictum, a fiber plant, is explored in the Tarim river inland delta but has a very small Kc value that results from low water availability due to upstream withdraw. Kc values are larger in the Liaoning region, where water is less limiting. Grass riparian vegetation is present in the Horqin sandy area, in Inner Mongolia, where groundwater is under the sandy dunes and showing in diverse small lakes; there, Chinese rye grass provides for pasture with a Kc mid value of 0.75. In all cases results show the Kc curves following the four segments FAO Kc curve.
The crop coefficients relative to the southern riparian vegetation of USA (Table 7), like the previously referred riparian areas in China, are mostly < 1.00 and generally smaller than those of wetlands in similar climates (Tables 3, 4 and 5). Likely, this happens because available water for this vegetation is insufficient to match full water requirements, i.e., evapotranspiration is water, non-energy, limited in most riparian areas. These regions are marked by aridity. Kc values for riparian vegetation in cold winter and hot summer climates are quite similar to those analysed before. Most cases of USA are from the Lower Colorado river basin where dominant vegetation consist of cottonwood, willow, mesquite, tamarisk, thus woody plants able to withdraw water from much larger depths than herbaceous, also present in wetlands.
Table 7 also includes riparian zones from other countries: The Tugai forest and associated shrubs of the Amudarya Valley of Turkmenistan, fen wetland in the lower River Gjern, in Jutland, Denmark, and the Fenéka pond, Kis-Balaton Lake, in Hungary. The Tugai forest has Populus euphratica as dominant and the main shrub is Halostachys capsica; the first has a high Kc avg due to favourable GW withdraw, while the latter is sparse throughout dryer areas. The fen in Jutland has diverse herbaceous vegetation with quite high Kc values since the area has much rainfall, quite distinct from the other referred riparian areas. The Hungarian study, is also from an area with relatively high rainfall, resulting that the vegetation, predominantly herbaceous, have a high Kc mid of 1.10. The European riparian areas result very distinct of the previously presented riparian ecosystems marked by aridity.
The riparian ecosystems relative to hot climates (Table 8), mostly in South USA, show Kc mid and Kc avg values generally smaller than 0.80, which reflect the occurrence of water stress since the vegetation has access to a limited amount of water that only partially satisfies its needs, as it is expected from riparian vegetation in areas with a large evaporation demand of the atmosphere. In the Low Colorado River basin and the San Pedro River valley, the more frequent dominant vegetation is Tamarix spp., Prosopis velutina, Populus spp., Salix spp. and, where vegetation is scarcer, the Pluchea sericea. Higher Kc values are for Populus fremonti but depending upon the water table depth.
Other selected papers refer to the Southern Hemisphere, namely the Silala river, in the border area between Bolivia and Chile, also arid, where mixed reed, grass, shrubs and hydrophytes show Kc avg = 0.62, also somewhat low. Three fruit trees—Ficus sycomorus, Philonoptera violacia and Diospyrus mespiliformis—are part of the riparian savanna by the Groot Letaba River, NE South Africa. The ecosystem Kc curve is similar to those of Mediterranean orchards (Pereira et al. 2023b). The site in New Zealand refers to a pasture close to a small stream with bordering trees and shrubs, which Kc curve, influenced by rainfall, is similar to those of grasslands reported by Pereira et al. (2023a). The cases for Australia refer to willows bordering two creeks that provide for abundant water, particularly during the initial and end-season stages, which make the segmented Kc curve to be inverted relative to the common one, as it happens with the olive in Mediterranean climates (Pereira et al. 2023b).
Table 9 presents the Kc values and selected characteristics of vegetation reported in the selected papers and that could be individualized. Related references are the same referred in Tables 7 and 8, as well as the online flora (http://www.worldfloraonline.org). The reported characteristics of the identified plants include the rivers regimes, permanent or temporary, and the groundwater table conditions: shallow, intermediate (1 to 5 m depth) and deep (5 m). It can be seen that vegetation identified as using permanent river water and/or shallow groundwater have larger Kc mid and/or Kc avg. Contrarily, plants having access to temporary river flow and or deeper water tables have smaller Kc. Nonetheless, tabulated Kc values are just related with the conditions reported in the selected papers, which may be different in other locations. Therefore, despite the Kc values tabulated were accurately derived, their values have to be assumed as indicative, to be used with caution until new research provides for better values. Anyway, they can be used for in the practice for computing plant evapotranspiration when an estimate of the FAO-PM ETo is available, as well as, to compute the water balance when other water outputs and inputs are known.
Conclusions
The current review study collected for the first-time crop coefficients reported in published research on evapotranspiration from riparian and non-riparian wetland vegetation ecosystems. This collection of information revealed consistent when comparing various types of wetlands and riparian ecosystems, particularly when comparing their Kc values. In fact, the collected Kc values are higher or lower according to the water availability recognizable through the papers descriptions and the water related characteristics of the identified plants and/or the online flora referred to in Tables 6 and 9. Considering the criteria used for collection of information as described in "Material and methods" section, the coherence observed in the tabulated data allows to assume confidence on the Kc values herein tabulated. The analysis and collection of Kc curves was not intended to be performed but may consist of future challenging research.
The tabulated Kc values are usable as default values for assessing the evapotranspiration of non-monitored wetland/riparian ecosystems and performing the respective water balances at various scales. For monitored ecosystems, the tabulated Kc may serve for comparing with locally collected data and, thus, testing the quality of related results. However, such use of the derived Kc requires the computation of the FAO-PM reference evapotranspiration since Kc values refer to this grass ETo. This comparison and test of results may be applied for whatever approach used for monitoring ET and the water balance. The simple computation of Kc from the fraction of ground cover and vegetation height, the A&P approach (Allen and Pereira 2009; Pereira et al. 2021), may be of great interest for monitoring wetlands and riparian ecosystems due to its simplicity and non-requiring but simple instrumentation. Research for improved and extended monitoring of ET and water balance of wetlands is nevertheless required.
Tabulated Kc values resulting from this review study may help understanding when evapotranspiration of a given ecosystem is water or energy limited and, consequently, may support decisions of water management authorities relative to the conservation or the betterment of wetlands, including riparian ecosystems. When the dynamics of ETc and of the water balance are known it is also easier to assess the ecosystems services, particularly those that relate with the water availability and to adopt appropriate water governance policies and measures,
It is well known that the search for water in regions marked by aridity led to the impoverishment and decrease of water available for life in riparian stands and wetlands. The quantification of water requirements using the Kc-ETo approach may better help to recognize the stress produced, specially under drought, and to define measures that shall avoid the progressive decline of wetlands and riparian zones. This is particularly relevant when aiming to control the appropriation of wetlands water for irrigation. Research aimed at understanding the role of water in wetlands and riparian ecosystems is much needed, namely in combination with water uses for irrigation, and mainly relative to the sustainability of communities practicing agriculture, recollection, and beneficiating of ecosystem services.
This study allows comparison of a wide number of wetland and riparian ecosystems, mainly in terms of vegetation and evapotranspiration crop coefficients. Such a comparison is important because although riparian and non-riparian wetland ecosystems may have great similarities, they also have important differences. Using the Kc – ETo approach for that purpose is likely appropriate. Moreover, the above innovation may be developed in combination for both ecosystems, while at the same time research may support ecosystem services.
Abbreviations
- ASCE-PM-ETr :
-
Alfalfa reference ETr calculated using an extension of the FAO56 Penman–Monteith equation
- ASTER-VI:
-
Vegetation index from ASTER satellite
- Avg.:
-
Average
- B-C:
-
Blaney–Criddle equation
- BREB:
-
Bowen ratio energy balance
- Capacit.:
-
Capacitance sensors
- DL:
-
Drainage lysimeters
- EB:
-
Energy balance
- EC:
-
Eddy covariance
- EVI:
-
Enhanced Vegetation Index
- FAO:
-
Food and Agriculture Organization
- FAO56:
-
Food and Agriculture Organization Irrigation and Drainage Paper 56 (1998)
- FAO-PM-ETo :
-
FAO56 Penman–Monteith standardized grass reference ETo equation
- FDR:
-
Frequency domain reflectometry
- FLUXNET:
-
Global network of micrometeorological flux measurement sites
- gravim.:
-
Gravimetric method
- Grow-seas:
-
Growing season
- GVMI:
-
Global Vegetation Moisture Index
- GW:
-
Groundwater
- GW Lys.:
-
Water table lysimeter
- HS:
-
Hargreaves–Samani equation
- HWB:
-
Field or catchment hydrologic water balance
- LAI:
-
Leaf area index
- Makk:
-
Makkink equation
- Med:
-
Mediterranean
- METRIC:
-
Energy balance model for mapping evapotranspiration with internalized calibration
- ML:
-
Mini or micro lysimeters
- MODIS:
-
Moderate resolution imaging spectroradiometer
- NDVI:
-
Normalized Difference Vegetation Index
- NOAA-AVHRR:
-
National Oceanic Atmospheric Administration-Advanced Very High Resolution Radiometer
- Non-grow seas:
-
Non-growing season
- Perm:
-
Permanent river flow regime
- PM-eq.:
-
Penman–Monteith combination equation
- PT:
-
Priestley–Taylor equation
- RS:
-
Remote sensing
- RSVI:
-
RS vegetation indices
- SEB:
-
Surface energy balance
- SEBAL:
-
Surface energy balance algorithm for land model
- SEBS:
-
Surface energy balance system model
- SF:
-
Sap flow
- SR:
-
Surface renewal
- Ssflow bed:
-
Sub-surface flow beds
- S-SEBI:
-
Simplified surface energy balance index
- SSS-ET:
-
Single-satellite-scene method
- SW:
-
Double source method of Shuttleworth and Wallace
- SWB:
-
Soil water balance
- SWC:
-
Soil water content
- Temp.:
-
Temporary river flow regime
- TDR:
-
Time domain reflectrometer
- TSM:
-
Two source model
- Trop:
-
Tropical climate
- VI:
-
Vegetation index
- VVR:
-
Vegetation vitality ratio
- Win.:
-
Winter
- WL:
-
Weighing lysimeter
- WTL:
-
Water table lysimeter
References
Adame MF, Pettit NE, Valdez D, Ward D, Burford MA, Bunn SE (2017) The contribution of epiphyton to the primary production of tropical floodplain wetlands. Biotropica 49(4):461–471
Allen RG (1998) Predicting evapotranspiration demand for wetlands. Paper to ASCE Wetlands Engineering Conf., ASCE, NY.
Allen RG, Pereira LS (2009) Estimating crop coefficients from fraction of ground cover and height. Irrig Sci 28:17–34
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop Evapotranspiration Guidelines for Computing Crop Water Requirements. FAO Irrig Drain Pap 56, FAO, Rome, p 300
Allen RG, Tasumi M, Morse A, Kranber WJ, Bastiaansen W (2005) Estimating agricultural and riparian ET through remote sensing in New Mexico. In: Aswathanarayana U (ed) Advances in water science methodologies. Balkema, Leyden, pp 73–90
Allen RG, Pruitt WO, Wright JL, Howell TA, Ventura F, Snyder R, Itenfisu D, Steduto P, Berengena J, Baselga J, Smith M, Pereira LS, Raes D, Perrier A, Alves I, Walter I, Elliott R (2006) A recommendation on standardized surface resistance for hourly calculation of reference ETo by the FAO56 penman-monteith method. Agric Water Manag 81:1–22
Allen RG, Tasumi M, Morse A, Trezza R, Wright JL, Bastiaanssen W, Kramber W, Lorite I, Robison CW (2007) Satellite-based energy balance for mapping evapotranspiration with internalized calibration (METRIC)—applications. J Irrig Drain Eng 133(4):395–406
Allen RG, Pereira LS, Howell TA, Jensen ME (2011) Evapotranspiration information reporting: II. Recommended documentation agricultural. Water Manag 98(6):921–929
Allen RG, Dhungel R, Dhungana B, Huntington J, Kilic A, Morton C (2021) Conditioning point and gridded weather data under aridity conditions for calculation of reference evapotranspiration. Agric Water Manag 245:106531
Anda A, Silva JAT, Soosa G (2014) Evapotranspiration and crop coefficient of common reed at the surroundings of lake Balaton, Hungary. Aquat Bot 116:53–59
Anda A, Soosa G, Silva JAT, Kozma-Bognara V (2015) Regional evapotranspiration from a wetland in central Europe, in a 16-year period without human intervention. Agric for Meteorol 205:60–72
Andersen HE, Hansen S, Jensen HE (2005) Evapotranspiration from a riparian fen wetland. Nord Hydrol 36(2):121–135
Baeumler NW, Kjaersgaard J, Gupta SC (2019) Evapotranspiration from corn, soybean, and prairie grasses using the METRIC model. Agron J 111:770–780
Barco A, Maucieri C, Borin M (2018) Root system characterization and water requirements of ten perennial herbaceous species for biomass production managed with high nitrogen and water inputs. Agric Water Manag 196:37–47
Bawazir AS, Samani Z, Bleiweiss M, Skaggs R, Schmugge T (2009) Using ASTER satellite data to calculate riparian evapotranspiration in the Middle RIO Grande, New Mexico. Int J Remote Sens 30(21):5593–5603
Bawazir AS, Luthy R, King JP, Tanzy BF, Solis J (2014) Assessment of the crop coefficient for saltgrass under native riparian field conditions in the desert southwest. Hydrol Process 28:6163–6171
Borin M, Milani M, Salvato M, Toscano A (2011) Evaluation of Phragmites australis (Cav.) Trin. evapotranspiration in northern and southern Italy. Ecol Eng 37(5):721–728
Buytaert W, Iñiguez V, Celleri R, De Bièvre B, Wyseure G, Deckers J (2006) Analysis of the water balance of small páramo catchments in South Ecuador. In: Krecek J, Haigh M (eds) Environmental role of wetlands in headwaters, vol 63. NATO Science Series, Springer, Dordrecht, pp 271–281
Carothers SW, Johnson RR, Finch DM, Kingsley KJ, Hamre, RH., tech eds (2020) Riparian Research and Management: Past, Present, Future. Vol 2, Gen. Tech. Rep. RMRS-GTR-411. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins. https://doi.org/10.2737/RMRS-GTR-411
Carrillo-Rojas G, Silva B, Rollenbeck R, Célleri R, Bendix J (2019) The breathing of the Andean highlands: net ecosystem exchange and evapotranspiration over the páramo of southern Ecuador. Agric for Meteorol 265:30–47
Chen P, Wang S, Liu Y, Wang Y, Wang Y, Zhang T, Zhang H, Yao Y, Song J (2023) Water availability in China’s oases decreased between 1987 and 2017. Earth’s Future 11:e2022EF003340
Chin DA (2011) Thermodynamic consistency of potential evapotranspiration estimates in Florida. Hydrol Process 25:288–301
Clulow AD, Everson CS, Mengistu MG, Jarmain CW, Jewitt GP, Price JS, Grundling P-L (2012) Measurement and modelling of evaporation from a coastal wetland in Maputaland, South Africa. Hydrol Earth Syst Sci 16:3233–3247
COUNCIL DIRECTIVE 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, THE COUNCIL OF THE EUROPEAN COMMUNITIES, https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0043:20070101:EN:PDF
Dadaser-Celik F, Stefan HG, Brezonik PL (2006) Dynamic hydrologic model of the Örtülüakar marsh in Turkey. Wetlands 26:1089–1102
Dai L, Fu R, Guo X, Ke X, Du Y, Zhang F, Li Y, Qian D, Zhou H, Cao G (2021) Evaluation of actual evapotranspiration measured by large-scale weighing lysimeters in a humid alpine meadow, northeastern Qinghai-Tibetan Plateau. Hydrol Process 35:e14051
de la Fuente B, Mateo-Sánchez MC, Rodríguez G, Gastón A, Pérez De Ayala R, Colomina-Pérez D, Melero M, Saura S (2018) Natura 2000 sites, public forests and riparian corridors: the connectivity backbone of forest green infrastructure. Land Use Policy 75:429–441
Dickard M, Gonzales M, Elmore W, Leonard S, Smith D, Smith S, Staats J, Summers P, Weixelman D, Wyman S (2015) Riparian area management - Proper functioning condition assessment for loticareas, p 199. BLM Technical Reference 1737–15. 2nd ed. U.S. Department of the Interior, Bureau of Land Management, National Operations Center, Denver, Colorado, USA
Dietrich O, Behrendt A, Wegehenkel M (2021) The water balance of wet grassland sites with shallow water table conditions in the North-Eastern German lowlands in extreme dry and wet years. Water 13:2259. https://doi.org/10.3390/w13162259
Dixon MJR, Loh J, Davidson NC, Beltrame C, Freeman R, Walpole M (2016) Tracking global change in ecosystem area: the wetland extent trends index. Biol Conserv 193:27–35
Doody TM, Benyon RG, Theiveyanathan S, Koul V, Stewart L (2014) Development of pan coefficients for estimating evapotranspiration from riparian woody vegetation. Hydrol Process 28:2129–2149
Doorenbos J, Pruitt WO (1977) Crop Water Requirements. FAO Irrig Drain Paper No 24 (rev), FAO, Rome
Drexler JZ, Anderson FE, Snyder RL (2008) Evapotranspiration rates and crop coefficients for a restored marsh in the Sacramento-San Joaquin Delta, California, USA. Hydrol Process 22:725–735
Dufour SA, Rodríguez-González PM, Laslier M (2019) Tracing the scientific trajectory of riparian vegetation studies: main topics, approaches and needs in a globally changing world. Sci Total Environ 653:1168–1185
EPA (2001) Types of wetlands. EPA 843-F-01-002b. US Office of Water. Environmental Protection Office of Wetlands, Agency Oceans and Watersheds (4502T)
Fermor PM, Hedges PD, Gilbert JC, Gowing DJG (2001) Reedbed transpiration rates in England. Hydrol Process 18:621–631
Flowers TJ, Colmer T (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115:327–331. https://doi.org/10.1093/aob/mcu267
Fontes JC, Dias E, Pereira LS (2006) Contributo da precipitação horizontal no balanço hidrológico em zonas altas com vegetação natural. In: IV Jornadas Forestales de la Macaronesia, Breña Baja, La Palma, Islas Canarias
Galbraith H; Amerasinghe P; Huber-Lee A (2005) The effects of agricultural irrigation on wetland ecosystems in developing countries: A literature review. CA Discussion Paper 1. Comprehensive Assessment Secretariat, Colombo (http://www.iwmi.cgiar.org/assessment)
Gazal RM, Scott RL, Goodrich DC, Williams DG (2006) Controls on transpiration in a semiarid riparian cottonwood forest. Agric Forest Meteorol 137:56–67
Gerling L, Weber TKD, Reineke D, Durner W, Martin S, Weber S (2019) Eddy covariance based surface-atmosphere exchange and crop coefficient determination in a mountainous peatland. Ecohydrology 12:e2047
Glenn EP, Neale CMU, Hunsaker DJ, Nagler PL (2011) Vegetation index-based crop coefficients to estimate evapotranspiration by remote sensing in agricultural and natural ecosystems. Hydrol Process 25:4050–4062
Glenn EP, Mexicano L, Garcia-Hernandez J, Nagler PL, Gomez-Sapiens MM, Tang D, Lomeli MA, Ramirez-Hernandez J, Zamora-Arroyo F (2013) Evapotranspiration and water balance of an anthropogenic coastal desert wetland: responses to fire, inflows and salinities. Ecol Eng 59:176–184
Gokool S, Jarmain C, Riddell E, Swemmer A, Lerm R Jr, Chetty KT (2017) Quantifying riparian total evaporation along the Groot Letaba River: a comparison between infilled and spatially downscaled satellite derived total evaporation estimates. J Arid Environ 147:114–124
Granath G, Moore PA, Lukenbach MC, Waddington JM (2016) Mitigating wildfire carbon loss in managed northern peatlands through restoration. Sci Rep 6:28498
Grismer ME (2018) Putah Creek hydrology affecting riparian cottonwood and willow tree survival. Environ Monit Assess 190:458
Gunawardhana M, Silvester E, Jones OAH, Grover S (2021) Evapotranspiration and biogeochemical regulation in a mountain peatland: insights from eddy covariance and ionic balance measurements. J Hydrol Reg Stud 36:100851
Guo H, Wang S, He X, Ding Y, Fan Y, Fu H, Hong X (2022) Characteristics of evapotranspiration and crop coefficient correction at a permafrost swamp meadow in dongkemadi watershed, the source of yangtze river in interior qinghai-tibet plateau. Water 14:3578
Hall BM, Rus DL (2013) Comparison of Water Consumption in Two Riparian Vegetation Communities along the Central Platte River, Nebraska, (2008–09 and 2011). US Geological Survey Scientific Investigations Report 2013–5203, https://doi.org/10.3133/sir20135203.
Hauenstein E, Peña-Cortés F, Bertrán C, Tapia J, Vargas-Chacoff L, Urrutia O (2014) Composición florística y evaluación de la degradación del bosque pantanoso costero de temupitra en la Región de La Araucanía, Chile (Floristic composition and evaluation of the degradation of the swampy coastal forest of temu-pitra in the Araucanía Region, Chile). Gayana Bot vol. 71n.1 https://doi.org/10.4067/S0717-66432014000100008
Hirota M, Kawada K, Hu Q, Kato T, Tang Y, Mo W, Cao G, Mariko S (2007) Net primary productivity and spatial distribution of vegetation in an alpine wetland, Qinghai-Tibetan plateau. Limnology 8:161–170
Hou LG, Xiao HL, Si JH, Xiao SC, Zhou MX, Yang YG (2010) Evapotranspiration and crop coefficient of Populus euphratica Oliv forest during the growing season in the extreme arid region northwest China. Agric Water Manag 97(2):351–356
Howes DJ, Fox P, Hutton PH (2015) Evapotranspiration from natural vegetation in the central valley of California: Monthly grass reference-based vegetation coefficients and the dual crop coefficient approach. J Hydrol Eng 20(10):04015004
Hu S, Niu Z, Chen Y, Li L, Zhang H (2017) Global wetlands: Potential distribution, wetland loss, and status. Sci Total Environ 586:319–327
Hughes CE, Kalma JD, Binning P, Willgoose GR, Vertzonis M (2001) Estimating evapotranspiration for a temperate salt marsh, newcastle, Australia. Hydrol Process 15:957–975
Hwang K, Chandler DG, Shaw SB (2020) Patch scale evapotranspiration of wetland plant species by ground-based infrared thermometry. Agric Forest Meteorol 287:107948
Ilhardt BL, Verry ES, Palik BJ (2000) Defining riparian areas. In: Wagner, RG, Hagan, JM (eds) Forestry and the Riparian Zone, Conf. Proc., Cooperative Forestry Research Unit, University of Maine
Irmak S, Kabenge I, Rudnick D, Knezevic S, Woodward D, Moravek M (2013) Evapotranspiration crop coefficients for mixed riparian plant community and transpiration crop coefficients for common reed, cottonwood and peach-leaf willow in the Platte River Basin, Nebraska-USA. J Hydrol 481:177–190
Jensen ME (2003) Vegetative and open water coefficients for the Lower Colorado River Accounting System (LCRAS). Addendum to the 1998 Report for the US Bureau of Reclamation, Boulder City, Nevada.
Jia L, Xi G, Liu S, Huang C, Yan Y, Liu G (2009) Regional estimation of daily to annual regional evapotranspiration with MODIS data in the yellow river delta wetland. Hydrol Earth Syst Sci 13:1775–1787
Jiao P, Hu S-J (2023) Estimation of evapotranspiration in the desert-oasis transition zone using the water balance method and groundwater level fluctuation method—taking the Haloxylon ammodendron forest at the edge of the Gurbantunggut Desert as an example. Water 15:1210
Johnson RR, Carothers SW, Finch DM, Kingsley KJ, Stanley JT, tech. eds. (2018) Riparian research and management: Past, present, future: Vol. 1. Gen. Tech. Rep. RMRS-GTR-377. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. https://doi.org/10.2737/RMRS-GTR-377
Kaletová T, Loures L, Castanho RA, Aydin E, Telo da Gama J, Loures A, Truchy A (2019) Relevance of intermittent rivers and streams in agricultural landscape and their impact on provided ecosystem services—a mediterranean case study. Int J Environ Res Public Health 16:2693
Khand K, Taghvaeian S, Esfahani LH (2017) Mapping annual riparian water use based on the Single-Satellite-Scene approach. Remote Sens 9:832. https://doi.org/10.3390/rs9080832
Landon MK, Rus DL, Dietsch BJ, Johnson MR, Eggemeyer KD (2009) Evapotranspiration Rates of Riparian Forests, Platte River, Nebraska, (2002–06). US Geological Survey Scientific Investigations Report 2008, 5228, Lincoln, Nebraska
Li F, Wang B (2015) A Study of fitting a swamp meadow ecosystem evapotranspiration to a model based on the penman-monteith equation. J Chem. https://doi.org/10.1155/2015/315708. (article ID 315708)
Li H, Yi J, Zhang J, Zhao Y, Si B, Hill RL, Cui L, Liu X (2015) Modeling of soil water and salt dynamics and its effects on root water uptake in Heihe arid wetland, Gansu, China. Water 7:2382–2401
Li Z, Gao P, Hu X, Yi Y, Pan B, You Y (2020) Coupled impact of decadal precipitation and evapotranspiration on peatland degradation in the zoige basin, China. Phys Geogr 41(2):145–168
Liu B, Zhao W, Chang X, Li S, Zhang Z, Du M (2010) Water requirements and stability of oasis ecosystem in arid region, China. Environ Earth Sci 59:1235–1244
Mamat A, Halik Ü, Rouzi A (2018) Variations of ecosystem service value in response to land-use change in the Kashgar region, Northwest China. Sustainability 10:200
Mandžukovski D, Čarni A, Sotirovski K (eds) (2021) Interpretative Manual of European Riparian Forests and Shrublands. Ss Cyril and Methodius University in Skopje, Hans Em Faculty of Forest Sciences Landscape Architecture and Environmental Engineering, Skopje
Mao LM, Bergman MJ, Tai CC (2002) Evapotranspiration measurement and estimation of three wetland environments in the upper St. Johns River Basin Florida. J Am Water Resour Assoc 38(5):1271–1285
Miller RL, Fujii R (2010) Plant community, primary productivity, and environmental conditions following wetland re-establishment in the Sacramento-San Joaquin Delta, California. Wetl Ecol Manag 18:1–16
Min J-H, Perkins DB, Jawitz JW (2010) Wetland-groundwater interactions in subtropical depressional wetlands. Wetlands 30:997–1006
Mitsch W, Gosselink J (2015) Wetlands, 5th edn. Wiley, New York
Mitsch WJ, Bernal B, Hernandez ME (2015) Ecosystem services of wetlands. Int J Biodiv Sci Ecosyst Serv Manag 11:1–4
Moffett KB, Wolf A, Berry JA, Gorelick SM (2010) Salt marsh–atmosphere exchange of energy, water vapor, and carbon dioxide: effects of tidal flooding and biophysical controls. Water Resour Res 46:W10525
Mohamed YA, Bastiaanssen WGM, Savenije HHG (2004) Spatial variability of evaporation and moisture storage in the swamps of the upper nile studied by remote sensing techniques. J Hydrol 289:145–164
Monteith JL (1965) Evaporation and environment. In: The state and movement of water in living organisms, 19th symp of soc exp biol, Cambridge University Press, Cambridge, pp 205–234
Moors EJ, Strieker JNM, van den Abeele GD (1998) Evapotranspiration of cut over bog covered by Molinia caerulea. Report 73 Afdeling Waterhuishouding, Wageningen
Nagler PL, Glenn EP, Nguyen U, Scott RL, Doody T (2013) Estimating riparian and agricultural actual evapotranspiration by reference evapotranspiration and MODIS enhanced vegetation index. Remote Sens 5:3849–3871
Nagler PL, Barreto-Muñoz A, Sall I, Lurtz MR, Didan K (2023) Riparian plant evapotranspiration and consumptive use for selected areas of the little Colorado river watershed on the Navajo Nation. Remote Sens 15:52
Nayak A, Bhushan B (2022) Wetland ecosystems and their relevance to the environment: importance of wetlands. In: Rathoure AK (ed) Handbook of Research on Monitoring and Evaluating the Ecological
Neale CMU, Geli H, Taghvaeian S, Masih A, Pack RT, Simms RD, Baker M, Milliken JA, O’Meara S, Witherall AJ (2011) Estimating evapotranspiration of riparian vegetation using high resolution multispectral, thermal infrared and lidar data. In: Neale CMU, Maltese A, Richter K (eds) Remote sensing for agriculture, ecosystems, and hydrology XIII, Proc of SPIE 8174, https://doi.org/10.1117/12.903246
Nichols J, Eichinger W, Cooper DI, Prueger JH, Hipps, LE, Neale CMU, Bawazir AS (2004) Comparison of evaporation estimation methods for a riparian area. Final Report. IIHR Technical Report No 436, College of Engineering, University of Iowa, Iowa City
Nouri M, Homaee M, Pereira LS, Bybordi (2023) Water management dilemma in the agricultural sector of Iran: a review focusing on water governance. Agric Water Manag 288:108480
Paredes P, Pereira LS, Almorox J, Darouich H (2020) Reference grass evapotranspiration with reduced data sets: parameterization of the FAO Penman-Monteith temperature approach and the Hargeaves-Samani equation using local climatic variables. Agric Water Manag 240:106210
Paredes P, Trigo I, de Bruin H, Simões N, Pereira LS (2021) Daily grass reference evapotranspiration with meteosat second generation shortwave radiation and reference ET products. Agric Water Manag 248:106543
Peacock CE, Hess TM (2004) Estimating evapotranspiration from a reed bed using the Bowen ratio energy balance method. Hydrol Process 18:247–260
Pereira LS, Paredes P, Melton F, Johnson L, Mota M, Wang T (2021) Prediction of crop coefficients from fraction of ground cover and height. Practical application to vegetable, field and fruit crops with focus on parameterization. Agric Water Manag 252:106663
Pereira LS, Paredes P, Espírito-Santo D, Salman M (2023a) Actual and standard crop coefficients for semi-natural and planted grasslands and grasses: a review aimed at supporting water management to improve production and ecosystem services. Irrig Sci. https://doi.org/10.1007/s00271-023-00867-6
Pereira LS, Paredes P, Oliveira CM, Montoya F, López-Urrea R, Salman M (2023b) Single and basal crop coefficients for estimation of water use of tree and vine woody crops with consideration of fraction of ground cover, height, and training system for mediterranean and warm temperate fruit and leaf crops. Irrig Sci. https://doi.org/10.1007/s00271-023-00901-7
Pôças I, Calera A, Campos I, Cunha M (2020) Remote sensing for estimating and mapping single and basal crop coefficients: A review on spectral vegetation indices approaches. Agric Water Manag 233:106081
Priestley CHB, Taylor RJ (1972) On the assessment of surface heat flux and evaporation using large-scale parameters. Mon Weather Rev 100(2):81–92
Rashed AA (2014) Assessment of aquatic plants evapotranspiration for secondary agriculture drains (case study: Edfina drain, Egypt). Egypt J Aquat Res 40:117–124
Read KE, Hedges PD, Fermor PM (2008) Monthly evapotranspiration coefficients of large reed bed habitats in the United Kingdom. In: Vymazal J (ed) Wastewater treatment, plant dynamics and management in constructed and natural wetlands. Springer, pp 99–109
Rebelo AJ, Jarmain C, Esler KJ, Cowling RM, Le Maitre DC (2020) Water-use characteristics of palmiet (Prionium serratum), an endemic South African wetland plant. Water SA 46(4):558–572
Ren D, Xu X, Ramos TB, Huang Q, Huo Z, Huang G (2017) Modeling and assessing the function and sustainability of natural patches in salt-affected agro-ecosystems: application to tamarisk (Tamarix chinensis Lour.) in Hetao, upper Yellow River basin. J Hydrol 552:490–504
Rosa RD, Paredes P, Rodrigues GC, Alves I, Fernando RM, Pereira LS, Allen RG (2012) Implementing the dual crop coefficient approach in interactive software 1: background and computational strategy. Agric Water Manag 103:8–24
Rose P (2021) Evidence for aviculture: identifying research needs to advance the role of ex situ bird populations in conservation initiatives and collection planning. Birds 2:77–95
Sanches L, Vourlitis GL, Alves MC, Pinto-Júnior OB, Nogueira JS (2011) Seasonal patterns of evapotranspiration for a Vochysia divergens forest in the Brazilian Pantanal. Wetlands 31:1215–1225
Sarika M, Zikos A (2020) Coastal salt marshes: structure and function of plant communities. In: Grigore MN (ed) Handbook of halophytes. Springer Nature, Switzerland AG. https://doi.org/10.1007/978-3-030-17854-3_7-1
Sarwar MW, Campbell DI, Shokri A (2022) Riparian zone as a variable source area for the estimation of evapotranspiration through the analysis of daily fluctuations in streamflow. Hydrol Proces 36:e14708. https://doi.org/10.1002/hyp.1470
Scott RL, Shuttleworth WJ, Goodrich DC, Maddock T III (2000) The water use of two dominant vegetation communities in a semiarid riparian ecosystem. Agric For Meteorol 105:241–256
Shafroth PB, Fuentes TL, Pritekel C, Beirne MM, Beauchamp VB (2020) Vegetation of the Elwha River Estuary. Chapter 8. In: Duda JJ, Warrick JA, Magirl CS (eds) Coastal habitats of the Elwha River, Washington—biological and physical patterns and processes prior to dam removal. US Geological Survey Scientific Investigations Report 5120, p. 225–248.
Shuttleworth WJ, Wallace JS (1985) evaporation from sparse crops—an energy combination theory. Q J Roy Meteor Soc 111:839–855
Šimůnek J, van Genuchten MTh, Šejna M (2016) Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J. 15(7):vzj2016040033
Solis JC, Bawazir AS, Tanzy BF, Luthy RG, Jeon S (2024) Inducing evapotranspiration reduction in an engineered natural system to manage saltcedar in riparian areas of arid environments. Water 16:53
Stannard DI, Gannett MW, Polette DJ, Cameron JM, Waibel MS, Spears JM (2013), Evapotranspiration from marsh and open-water sites at Upper Klamath Lake, Oregon, (2008–2010. US Geological Survey Scientific Investigations Report 2013–5014
Stella JC, Rodríguez-González PM, Dufour S, Bendix J (2013) Riparian vegetation research in mediterranean-climate regions: common patterns, ecological processes, and considerations for management. Hydrobiol 719:291–315
Suárez F, Oportus T, Mendoza M, Aguirre I, Godoy V, Muñoz JF (2023) Evaporation processes in the Silala River basin. Wires Water. https://doi.org/10.1002/wat2.1638
Sueltenfuss JP, Cooper DJ, Knight RL, Waskom RM (2013) The creation and maintenance of wetland ecosystems from irrigation canal and reservoir seepage in a semi-arid landscape. Wetlands 33:799–810
Thevs N, Rouzi A (2015) Evapotranspiration of cotton, apocynum pictum, and Zyzyphus jujuba in the Tarim basin, Xinjiang, China. J Water Resour Protect 7:1001–1009
Thevs N, Peng H, Rozi A, Zerbe S, Abdusalih N (2014) Water allocation and water consumption of irrigated agriculture and natural vegetation in the Aksu-Tarim river basin, Xinjiang, China. J Arid Environ 112(Part A):87–97
Thevs N, Ovezmuradov K, Zanjani LV, Zerbe S (2015) Water consumption of agriculture and natural ecosystems at the Amu Darya in Lebap province, Turkmenistan. Environ Earth Sci 73:731–741
Thevs N, Rouzi A, Aliev K, Abudushalike N (2017) Evapotranspiration of riparian ecosystems and irrigated cotton agriculture at the middle reaches of the Tarim river, Xinjiang, China. Central Asian J Water Res 3(4):1–15
UNESCO (1994) Convention on Wetlands of International Importance especially as Waterfowl Habitat. Ramsar 1971, amended 1982, 1987, 1994. Unesco, Paris. https://www.ramsar.org/sites/default/files/documents/library/current_convention_text_e.pdf
Urbanič G, Politti E, Rodríguez-González PM et al (2022) Riparian zones—from policy neglected to policy integrated. Front Environ Sci 10:868527
Verones F, Saner D, Pfister S, Baisero D, Rondinini C, Hellweg S (2013) Effects of consumptive water use on biodiversity in wetlands of international importance. Environ Sci Technol 47:12248–12257
Verry ES, Dolloff CA, Manning ME (2004) Riparian ecotone: a functional definition and delineation for resource assessment. Water Air Soil Poll: Focus 4(1):67–94
Wang H, Tetzlaff D, Soulsby C (2017) Testing the maximum entropy production approach for estimating evapotranspiration from closed canopy shrubland in a low-energy humid environment. Hydrol Proces 31:4613–4621
Westenburg CL, Harper DP, DeMeo GA (2006) Evapotranspiration by phreatophytes along the lower Colorado River at Havasu National Wildlife Refuge, Arizona: US Geological Survey Scientific Investigations Report 2006–5043, Reston, Virginia, 44 p.
Wu C-L, Shukla S (2014) Eddy covariance-based evapotranspiration for a subtropical wetland. Hydrol Process 28:5879–5896
Wu Y, Liu T, Paredes P, Duan L, Wang H, Wang T, Pereira LS (2016) Ecohydrology of groundwater-dependent grasslands of the semi-arid Horqin sandy land of inner Mongolia focusing on evapotranspiration partition. Ecohydrol 9:1052–1067
Xu T, Weng B, Yan D, Wang K, Li X, Bi W, Li M, Cheng X, Liu Y (2019) Wetlands of international importance: status, threats, and future protection. Int J Environ Res Public Health 16:1818
Xu X, Chen M, Yang G, Jiang B, Zhang J (2020) Wetland ecosystem services research: a critical review. Global Ecol Conserv 22:e01027
Yang Y, Chen R, Song Y, Han C, Liu J, Liu Z (2017) Actual daily evapotranspiration and crop coefficients for an alpine meadow in the Qilian Mountains, Northwest China. Hydrol Res 484:1131–1142
Yao M-H, Li M-H, Juan J-Y, Hsia Y-J, Lee P-H, Shen Y (2017) Mapping reference evapotranspiration from meteorological satellite data and applications. Terr Atmos Ocean Sci 28(3):501–515
Yu GA, Disse M, Huang HQ, Yu Y, Li ZW (2016) River network evolution and fluvial process responses to human activity in a hyper-arid environment—case of the Tarim River in Northwest China. CATENA 147:96–109
Yu T, Qi F, Si J, Zhang X, Zhao C (2017) Tamarix ramosissima stand evapotranspiration and its association with hydroclimatic factors in an arid region in Northwest China. J Arid Environ 138:18–26
Yu T, Qi F, Si J, Zhang X, Xi H, Zhao C (2018) Comparable water use of two contrasting riparian forests in the lower Heihe River basin, Northwest China. J For Res 29(5):1215–1224
Yuan G, Zhang P, Shao M, Luo Y, Zhu X (2014) Energy and water exchanges over a riparian Tamarix spp. stand in the lower Tarim River basin under a hyper-arid climate. Agric For Meteorol 194:144–154
Yuan G, Zhu X, Tang X, Du T, Yi X (2016) A species-specific and spatially-explicit model for estimating vegetation water requirements in desert riparian forest zones. Water Resour Manage 30:3915–3933
Yue W, Wang T, Franz TE, Chen X (2016) Spatiotemporal patterns of water table fluctuations and evapotranspiration induced by riparian vegetation in a semiarid area. Water Resour Res 52:1948–1960
Yuguda TK, Wu Y, Leng Z, Gao G, Li G, Dai Z, Li J, Du D (2022) Impact of Spartina alterniflora invasion on evapotranspiration water loss in Phragmites australis dominated coastal wetlands of east China. Ecol Eng 179:106605
Zaimes G, Nichols M, Green D, Crimmins M (2007) Understanding Arizona's Riparian Areas. College of Agriculture and Life Sciences, University of Arizona, Tucson, http://hdl.handle.net/10150/146921
Zeng Y, Zhao Ch, Shi F, Schneider M, Guanghui L, Yan L (2020) Impact of groundwater depth and soil salinity on riparian plant diversity and distribution in an arid area of China. Sci Rep 10:7272. https://doi.org/10.1038/s41598-020-64045-w
Zhao L, Zhao W (2014) Evapotranspiration of an oasis-desert transition zone in the middle stream of Heihe River, Northwest China. J Arid Land 6(5):529–539
Zheng X, Zhu JJ, Yan QL, Song LN (2012) Effects of land use changes on the groundwater table and the decline of Pinus sylvestris var. mongolica plantations in southern Horqin Sandy Land, Northeast China. Agric Water Manag 109:94–106
Zhou L, Zhou G (2009) Measurement and modelling of evapotranspiration over a reed (Phragmites australis) marsh in Northeast China. J Hydrol 372:41–47
Acknowledgements
The support of the FCT, Fundação para a Ciência e a Tecnologia, I.P., under the project UIDB/04129/2020 of LEAF-Linking Landscape, Environment, Agriculture and Food, Research Unit, and to support P. Paredes (https://doi.org/https://doi.org/10.54499/DL57/2016/CP1382/CT0022) are acknowledged. The study was also funded through an agreement between FAO, Food and Agricultural Organization of the United Nations, and the Instituto Superior de Agronomia, Universidade de Lisboa.
Funding
Open access funding provided by FCT|FCCN (b-on). Fundação para a Ciência e a Tecnologia, UIDB/04129/2020, FAO, Food and agriculture Organization, FAO-ISA-RP- 355071.
Author information
Authors and Affiliations
Contributions
LSP and PP designed and contributed to the search and selection of the reviewed articles; LSP performed the writing, and all authors revised the article. DES revised the botanical issues and tabulation. All authors agreed on the submitted version of the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, L.S., Paredes, P. & Espírito-Santo, D. Crop coefficients of natural wetlands and riparian vegetation to compute ecosystem evapotranspiration and the water balance. Irrig Sci (2024). https://doi.org/10.1007/s00271-024-00923-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00271-024-00923-9