Abstract
Purpose
To describe clinical outcomes among patients with benign prostatic hyperplasia (BPH) 24 months following prostatic artery embolization (PAE).
Materials and Methods
This was an international, multicenter, prospective trial of males with BPH with lower urinary tract symptoms (LUTS) or acute urinary retention (AUR) treated with PAE. The primary outcome was the 12 month change in the International Prostate Symptom Score (IPSS) for patients referred for bothersome LUTS, or urinary catheter independence for patients treated for AUR. Secondary outcome measures included changes in IPSS at 3 and 24 months, changes in quality of life (QoL), changes in the Sexual Health Inventory for Men (SHIM) questionnaire, technical success rate, and adverse events (AEs). Data were summarized using descriptive statistics.
Results
Four hundred seventy-eight consecutive patients underwent PAE (bothersome LUTS: N = 405; AUR: N = 73), mean age was 70 years. For patients treated for bothersome LUTS, mean total IPSS at baseline was 21.8 and decreased to 9.3, 10.6, and 11.2 at 3, 12, and 24 months following PAE, respectively (all p < 0.001); QoL at baseline was 4.7 and decreased to 2.0, 2.1, and 2.3 at 3, 12, and 24 months, respectively (all p < 0.001). The mean SHIM score at baseline and 12 months following PAE was 13.8 and 13.9, respectively. Of the 73 patients treated for AUR, 48 (65.8%) had their indwelling catheter removed within 3 months of PAE and remained catheter free at 24 months. Fifty-five patients (11.5%) experienced ≥ 1 AE and 10 (2.1%) experienced a serious AE.
Conclusion
PAE is a safe and effective treatment for symptomatic BPH and LUTS.
Level of Evidence Level 3
Trial registration ClinicalTrials.gov NCT03527589.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH) is a common urological condition that affects men [1]. For patients unresponsive to lifestyle modifications and pharmacotherapy, guidelines recommend surgical treatment [2]. Transurethral resection of the prostate (TURP) is the standard surgical therapy for BPH [3]; however, newer surgical therapies (e.g., Urolift, Rezum) have shown promise as minimally invasive treatments that can help avoid complications (e.g., bleeding, incontinence, sexual dysfunction associated with TURP) [2]. Prostatic artery embolization (PAE) is a newer minimally invasive technique that causes partial ischemic necrosis of the prostate gland and softening of the gland that can lead to reduction in BPH and symptomatic improvement [4, 5].
Several clinical trials, cohort studies, reviews, and meta-analyses evaluating clinical outcomes following PAE and other therapies for BPH with lower urinary tract symptoms (LUTS) have been published [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. A common finding in the clinical trial setting is that PAE is an effective therapy that is associated with a generally high rate of technical success. Among trials comparing outcomes following PAE versus TURP, patients that underwent embolization typically experienced similar clinical improvement as those treated with TURP but with fewer adverse events (AEs) [6, 8, 10, 27, 28]. In clinical practice, PAE is considered to be a viable alternative for BPH management, particularly among patients that are unable or refuse surgery [15, 16, 18,19,20, 29].
As several prior studies have highlighted the safety and effectiveness of PAE, the goal of this study was to provide additional real-world evidence describing clinical outcomes following the procedure. An important aspect of this study was that it captured the safety and effectiveness profiles of PAE across multiple international centers and operators over 24 months.
Methods
Study Design
This was a prospective cohort registry study conducted across 14 centers in France, Italy, the United Kingdom, and the United States.
The inclusion criteria included patients over 18 years of age with symptomatic BPH or acute urinary retention (AUR), who were willing to undergo PAE and provided informed consent. Patients were excluded if they were unable or unwilling to provide follow-up information, were undergoing PAE for reasons unrelated to symptomatic LUTS due to BPH, or any other reason the investigator deemed cause for exclusion (e.g. significant comorbidities preventing the patient from lying flat and still). The criteria for inclusion in the study were broad to capture a range of outcomes among a diverse patient population.
Study Cohorts
This study included two patient cohorts: those with BPH-related bothersome LUTS but without an indwelling bladder catheter (LUTS cohort) and those with AUR due to underlying BPH with a urinary bladder catheter (AUR cohort).
PAE Procedure
All procedures were performed via femoral or radial access according to standard practices at each participating center. In general, the microcatheter was advanced into the prostatic artery using a road mapping technique. The prostatic artery was embolized using 100–300 µm or 300–500 µm Embosphere® Microspheres (Merit Medical, South Jordan, Utah, USA) until total arterial occlusion occurred. Each vial of microspheres was diluted up to 20 mL with a mixture of 50/50 contrast and saline. Most interventions were performed as a same-day outpatient procedure under local anesthesia with or without moderate sedation.
Outcome Measures
The primary outcome measure was the 12 month change in the International Prostate Symptom Score (IPSS). Secondary outcome measures included: (1) IPSS measurements at 3 and 24 months; (2) device-related and procedure-related AEs at 3, 12, and 24 months following PAE; (3) technical success (i.e., technically successful embolization of at least one prostatic artery); (4) removal of the indwelling catheter in those treated for AUR; (5) number of patients with refractory or recurrent LUTS due to BPH at 3, 12, and 24 months post-PAE; and (6) 12 month changes in erectile function, assessed using the Sexual Health Inventory for Men (SHIM) questionnaire.
Clinical characteristics assessed included prostate size, maximum urinary flow rate (Qmax), post-void residual (PVR) volume, and prescribed prostate medications. Procedural characteristics assessed included volume of embolic administered, unilateral versus bilateral embolization, and procedure time.
Treatment-related AEs were reviewed and adjudicated based on the clinical judgement of an independent physician with experience in PAE. AEs were considered to be serious if they met any of the following criteria: (1) resulted in death; (2) were life-threatening; (3) required in-patient hospitalization or prolongation of existing hospitalization; (4) resulted in persistent or significant disability/incapacity; (5) were considered an important medical event, which was defined as an event that may not result in death, be life-threatening, or necessitate hospitalization but, based on discretion of the medical staff, may jeopardize the patient and/or necessitate medical or surgical intervention.
Statistical Analysis
Continuous data were summarized using mean and standard deviation. Categorical variables were summarized using frequency, counts, and percentages. Technical parameters were reported as the proportion of patients that underwent unilateral or bilateral PAE. Relative changes in IPSS and quality of life (QoL) over the follow-up period were evaluated using independent t-tests and reported as mean changes with standard deviations. Changes in SHIM from baseline to 12 months following PAE were reported as means and standard deviations; p values < 0.05 were considered significant.
Results
Patient Demographics and Procedural Characteristics
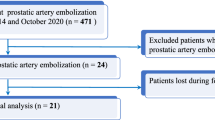
Of the 488 patients enrolled, 10 were excluded from analysis due to lost to follow up (n = 1) and PAE not attempted/completed due to challenging anatomy (n = 9) (Fig. 1). The mean age of all patients was 70 ± 8 years; patients in the LUTS cohort were significantly younger than patients in the AUR cohort (69 ± 8 vs. 75 ± 10 years, p < 0.0001).
Of the 478 patients that underwent PAE, 405 (84.7%) were for bothersome LUTS and 73 (15.3%) were for AUR (Fig. 1). The mean volume of diluted microspheres administered was 13.0 ± 6.9 mL (LUTS cohort: 13.0 ± 7.0; AUR cohort: 12.6 ± 6.6). The mean PAE procedure time was 111.1 ± 45.3 min overall (LUTS cohort: 109.5 ± 45.5; AUR cohort: 120.1 ± 44.2).
Primary Outcome Measure
Among the 405 patients with bothersome LUTS, the 12 month relative change in IPSS was − 11.1 ± 8.3 (p < 0.001; Table 1). The mean IPSS at baseline was 21.8 ± 6.6 and 12 months post-PAE, the mean IPSS was 10.6 ± 7.5 (Table 1; Fig. 2). For patients in the AUR cohort, the mean IPSS was 7.7 ± 5.3; as no baseline IPSS data were available for the AUR cohort, the relative change could not be calculated.
Changes in IPSS, QoLa, and clinical characteristics of patients with bothersome LUTS. IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; PVR, post-void residual; Qmax, maximum urinary flow rate; QoL, quality of life; SHIM, Sexual Health Inventory for Men aQoL score is based on a single question within the IPSS assessment that asks patients “If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?”, scores are graded based on the following 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed about equally satisfied and dissatisfied), 5 (mostly dissatisfied), and 6 (terrible)
Secondary Outcome Measures
Technical success was achieved in all patients (Fig. 1). In both cohorts, most patients underwent bilateral embolization (LUTS cohort: 91.6%; AUR cohort: 80.8%). Among patients in the LUTS cohort, 34 (8.4%) underwent unilateral embolization compared to 14 (19.2%) patients in the AUR cohort.
In the bothersome LUTS cohort, the mean IPSS at 3 months was 9.3 ± 6.6 and the mean IPSS at 24 months was 11.2 ± 7.9. The relative changes in IPSS at 3 and 24 months post-PAE were − 12.4 ± 7.8 and − 10.2 ± 8.5, respectively, for the LUTS cohort (p < 0.001 for both time points). The mean SHIM scores at baseline and 12 months post-PAE were 13.8 ± 8.5 and 13.9 ± 8.8, respectively (relative change: − 0.04 ± 6.5; p = 0.912). The relative changes in QoL at 3, 12, and 24 months post-PAE were − 2.7 ± 1.7, − 2.6 ± 1.7, and − 2.3 ± 1.8, respectively (all p < 0.001). The relative change in prostate size was − 28.8 ± 32.7g at 3 months (p < 0.001), − 6.7 ± 30.6 g at 12 months (p = 0.428), and − 20.8 ± 33.5 g at 24 months (p = 0.304). The relative change in PVR volume was − 34.5 ± 104.3 mL at 3 months (p < 0.001), − 40.4 ± 86.8 mL at 12 months (p < 0.001), and − 34.5 ± 81.6 mL at 24 months (p = 0.015). The relative changes in Qmax were 4.9 ± 10.6 mL/s at 3 months (p < 0.001), 5.3 ± 9.3 mL/s at 12 months (p < 0.001), and 2.4 ± 10.4 mL/s at 24 months (p = 0.197). The mean values for clinical characteristics across the 24 month follow-up period are provided in Table 1.
Of the 73 patients in the AUR cohort, 48 patients (65.8%) had their indwelling bladder catheter removed within 3 months following PAE and remained catheter free during the study. The mean SHIM scores at baseline and 12 months post-PAE for the AUR cohort were 11.2 ± 10.0 and 9.8 ± 8.7, respectively (relative change: − 1.4 ± 7.8; p = 0.431). The relative change in prostate size was − 10.6 ± 21.8 g at 3 months (p = 0.339) and − 6.6 ± 39.1 g at 12 months (p = 0.727). The relative change in PVR volume was 10.8 ± 53.5 mL at 3 months (p = 0.715).
Of the 478 patients in the study, 16 (3.3%) underwent re-embolization following the initial PAE procedure due to secondary clinical failure. Of these 16 patients, 12 with bothersome LUTS underwent re-embolization within 24 months (n = 8 within 12 months, n = 4 within 24 months) of the initial procedure and 4 patients with AUR at baseline underwent re-embolization within 12 months of the initial procedure (none within 24 months). Twenty-four months post-PAE, 34.7% (17/49) of patients with AUR were still using BPH medications (n = 7: α-blockers only; n = 4: 5-α reductase inhibitors only; n = 6: ≥ 2 combined medications), and 34.3% (106/309) with bothersome LUTS (n = 69 α-blockers only; n = 9 5-α reductase inhibitors only; n = 28 ≥ 2 combined medications). Over the 24 month follow-up period, 32 of the 478 patients (6.7%) underwent surgery or a minimally invasive surgical therapy after 14.5 ± 9.3 months (n = 26 within 12 months, n = 6 within 24 months). Of these 32 patients, 24 were from the LUTS cohort (n = 19 at 12 months, n = 5 at 24 months) and 8 were from the AUR cohort (n = 7 at 12 months, n = 1 at 24 months).
Adverse Events
A total of 55 out of 478 (11.5%) patients had ≥ 1 AE (Table 2), with 10 (2.1%) experiencing a serious AE (Table 3). The most common AE was self-limiting irritative symptoms (21.5%; 103/478) (Table 2). One patient experienced a penile ulceration that resolved. One patient developed a rectoprostatic fistula following PAE for bothersome LUTS, this patient had a prior history of prostate cancer and underwent radiation therapy prior to PAE. The patient underwent conservative medical management for the fistula.
Discussion
Across all timepoints evaluated in this study, the mean changes in IPSS and QoL scores suggest durability of the clinical benefits associated with PAE. Additionally, the low rates of AEs and serious AEs observed during the follow-up period suggest that PAE is a safe option to address the clinical needs of this patient population.
The significant changes in IPSS and QoL scores relative to baseline among patients in this study align with prior studies [11, 14, 15]. In an observational study of men with LUTS [14], the differences in IPSS and QoL 12 months following PAE relative to baseline were − 10.9 and − 2.6, respectively, which match the − 11.1 and − 2.6 changes in IPSS and QoL scores, respectively, that were observed in the present study.
Definitions of clinical success following PAE vary across studies [4, 17, 30]. A commonly used definition for patients with bothersome LUTS is an IPSS of ≤ 15 and/or a ≥ 25% decrease in IPSS relative to baseline [17, 30]; for patients with AUR, clinical success is defined as the removal of the indwelling catheter. In this study, clinical success was not determined a priori. However, in the LUTS cohort 79.3% (242/305) of patients had an IPSS of ≤ 15 or a ≥ 25% decrease in IPSS at 24 months. This aligns with prior studies reporting clinical success rates of 82–90%. A total of 65.8% (48/73) of patients with AUR were able to have their indwelling catheter removed within 3 months of embolization and remained catheter free for the remainder of the 24 month follow-up period. This finding is slightly lower than other studies [16, 21] that reported indwelling catheter removal in 73–75% of patients following PAE. However, due to the small sample sizes in prior studies (n = 20 [21] and n = 26 [16]), the proportion of patients in this study that were catheter-free following embolization may be comparable.
In the present study, 16 (3.3%) patients were re-embolized within 24 months, 32 (6.7%) underwent surgery or another minimally invasive procedure. Approximately one-third of patients in both cohorts were still using medications to manage their BPH, this proportion aligns with a prior study that reported 31% of patients using BPH medications following PAE [31]. Although PAE is known to be an effective therapy for BPH with LUTS, it is not uncommon for some patients to require additional treatment due to recurrence of symptoms or inadequate response to the initial PAE procedure [14, 17, 32, 33]. Despite some variation in the proportion of patients that require re-intervention or continued medication, PAE is still considered an effective therapy with a durable response in most patients.
PAE is considered a safe procedure; serious complications are rare [34]. One study [4] reported a major complication rate of 1.6%, which aligns with the 2.1% reported in the present study. In this study, the occurrence of non-target embolization was low: one patient experienced penile ulceration, which was reversible, and aligns with prior reports of low rates of non-target embolization [4, 14].
In this study, there was a noticeable difference in the proportion of patients that underwent unilateral embolization (8% in the LUTS cohort vs. 19% in the AUR cohort). A potential reason for this discrepancy may be the significant difference in age as older patients have more atheromatous arteries that can sometimes preclude access to the prostatic artery. Nevertheless, the proportion of patients in both cohorts that experienced technical success of the procedure was high and aligns with prior evidence demonstrating the technical feasibility of the procedure and its clinical utility [35, 36]. Moreover, the clinical benefits and tolerable safety profile associated with PAE have been recognized by the Society for Interventional Radiology and the American Urological Association [37, 38]. As additional evidence demonstrating the tolerable safety and efficacy profiles associated with PAE emerge, future studies evaluating what, if any, impact heterogenous techniques have on outcomes will be an important consideration to address.
This study should be considered within the context of certain limitations. First, missing patient data due to patients lost to follow up (e.g., missed appointments) resulted in the inability to evaluate data for every patient across all timepoints. Second, without a control cohort, comparisons could not be made regarding the magnitude of improvement for untreated patients, or patients treated with other surgical procedures. Third, although 478 patients is an acceptable sample size for real-world data, no hypothesis testing was performed across all timepoints for both cohorts; therefore, we are unable to confirm whether the mean changes in all study measures relative to baseline were significant. Finally, this study was unable to capture the full range of reasons for subsequent procedures or reasons for loss of follow-up.
In conclusion, findings from this multicenter, international, prospective, cohort study of patients with BPH and bothersome LUTS, or AUR, who underwent PAE provide further evidence supporting the clinical utility of PAE. The generally low AE rates may encourage broader use in these patients.
Data Availability
Reasonable requests for anonymized data that support the findings of this work can be made to the corresponding author.
References
Vuichoud C, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol. 2015;22(Suppl 1):1–6.
Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART II-surgical evaluation and treatment. J Urol. 2021;206(4):818–26.
Bortnick E, Brown C, Simma-Chiang V, Kaplan SA. Modern best practice in the management of benign prostatic hyperplasia in the elderly. Ther Adv Urol. 2020;12:1756287220929486.
Carnevale FC, Moreira AM, de Assis AM, et al. Prostatic artery embolization for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: 10 years’ experience. Radiology. 2020;296(2):444–51.
de Assis AM, Moreira AM, Carnevale FC, et al. Role of ultrasound elastography in patient selection for prostatic artery embolization. J Vasc Interv Radiol. 2021;32(10):1410–6.
Abt D, Hechelhammer L, Mullhaupt G, et al. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ. 2018;361: k2338.
Gao YA, Huang Y, Zhang R, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate–a prospective, randomized, and controlled clinical trial. Radiology. 2014;270(3):920–8.
Carnevale FC, Iscaife A, Yoshinaga EM, et al. Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective. Urodyn-Controll Anal Cardiovasc Interven Radiol. 2016;39(1):44–52.
Russo GI, Kurbatov D, Sansalone S, et al. Prostatic arterial embolization versus open prostatectomy: a 1-year matched-pair analysis of functional outcomes and morbidities. Urology. 2015;86(2):343–8.
Insausti I, de Ocáriz AS, Galbete A, Capdevila F, Solchaga S, Giral P, Bilhim T, Isaacson A, Urtasun F, Napal S. Randomized comparison of prostatic artery embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. J Vasc Int Radiol. 2020;31(6):882–90. https://doi.org/10.1016/j.jvir.2019.12.810.
Tapping CR, Little MW, Macdonald A, et al. The STREAM Trial (prostatic artery embolization for the treatment of benign prostatic hyperplasia) 24 month clinical and radiological outcomes. Cardiovasc Interven Radiol. 2021;44(3):436–42.
Sapoval M, Thiounn N, Descazeaud A, et al. Prostatic artery embolisation versus medical treatment in patients with benign prostatic hyperplasia (PARTEM): a randomised, multicentre, open-label, phase 3, superiority trial. Lancet Reg Health Eur. 2023;31: 100672.
Pisco JM, Bilhim T, Costa NV, et al. Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. Eur Urol. 2020;77(3):354–62.
Ray AF, Powell J, Speakman MJ, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. 2018;122(2):270–82.
Qiu Z, Zhang C, Wang X, et al. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Wideochir Inne Tech Maloinwazyjne. 2017;12(4):409–16.
Salvador Hidalgo D, Bernardello Ureta M, Sbriglio M, et al. Prostatic artery embolization treatment for patients with benign prostatic hyperplasia who are permanent urinary catheter users ineligible for de-obstructive surgery. Actas Urol Esp (Engl Ed). 2021;45(7):481–5.
Pisco JM, Bilhim T, Pinheiro LC, et al. Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. J Vasc Interv Radiol. 2016;27(8):1115–22.
Rampoldi A, Barbosa F, Secco S, et al. Prostatic artery embolization as an alternative to indwelling bladder catheterization to manage benign prostatic hyperplasia in poor surgical candidates. Cardiovasc Interv Radiol. 2017;40(4):530–6.
Wang M, Guo L, Duan F, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms as a result of large benign prostatic hyperplasia: a prospective single-center investigation. Int J Urol. 2015;22(8):766–72.
Bhatia S, Sinha VK, Harward S, et al. Prostate artery embolization in patients with prostate volumes of 80 mL or more: a single-institution retrospective experience of 93 patients. J Vasc Interv Radiol. 2018;29(10):1392–8.
Kenny AG, Pellerin O, Amouyal G, et al. prostate artery embolization in patients with acute urinary retention. Am J Med. 2019;132(11):e786–90.
Franco JV, Jung JH, Imamura M, et al. Minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia: a network meta-analysis. Cochrane Database Syst Rev. 2021;7(7):CD013656.
Jung JH, McCutcheon KA, Borofsky M, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2022;3(3):CD012867.
Xu Z, Zhou Z, Mu Y, et al. An updated meta-analysis of the efficacy and safety of prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia. Front Surg. 2021;8:779571.
Lucas-Cava V, Sanchez-Margallo FM, Insausti-Gorbea I, Sun F. Comparative efficacy and safety of prostatic urethral lift versus prostatic artery embolization for benign prostatic hyperplasia: a systematic review and network meta-analysis. BJU Int. 2022;131(2):139–52.
Uflacker A, Haskal ZJ, Bilhim T, et al. Meta-analysis of prostatic artery embolization for benign prostatic hyperplasia. J Vasc Interv Radiol. 2016;27(11):1686-1697.e8.
Xiang P, Guan D, Du Z, et al. Efficacy and safety of prostatic artery embolization for benign prostatic hyperplasia: a systematic review and meta-analysis of randomized controlled trials. Eur Radiol. 2021;31(7):4929–46.
Knight GM, Talwar A, Salem R, Mouli S. Systematic review and meta-analysis comparing prostatic artery embolization to gold-standard transurethral resection of the prostate for benign prostatic hyperplasia. Cardiovasc Interv Radiol. 2021;44(2):183–93.
Grosso M, Balderi A, Arno M, et al. Prostatic artery embolization in benign prostatic hyperplasia: preliminary results in 13 patients. Radiol Med. 2015;120(4):361–8.
Frandon J, Belaouni A, Pellerin O, et al. Efficacy and safety of prostate artery embolization for patients with lower urinary tract symptoms and indwelling urinary catheter: a retrospective multicenter study. Diagn Interv Imaging. 2022;103(12):601–6.
Bilhim T, Costa NV, Torres D, Pinheiro LC, Spaepen E. Long-term outcome of prostatic artery embolization for patients with benign prostatic hyperplasia: single-centre retrospective study in 1072 patients over a 10-year period. Cardiovasc Interv Radiol. 2022;45(9):1324–36.
Abt D, Mullhaupt G, Hechelhammer L, et al. Prostatic artery embolisation versus transurethral resection of the prostate for benign prostatic hyperplasia: 2-yr outcomes of a randomised, open-label. Single-centre Trial Eur Urol. 2021;80(1):34–42.
Ayyagari R, Powell T, Staib L, et al. Prostatic artery embolization using 100–300-mum trisacryl gelatin microspheres to treat lower urinary tract symptoms attributable to benign prostatic hyperplasia: a single-center outcomes analysis with medium-term follow-up. J Vasc Interv Radiol. 2020;31(1):99–107.
Naidu SG, Narayanan H, Saini G, et al. Prostate artery embolization—review of indications, patient selection, techniques and results. J Clin Med. 2021;10(21):5139. https://doi.org/10.3390/jcm10215139.
Amouyal G, Tournier L, De Margerie-Mellon C, et al. Safety profile of ambulatory prostatic artery embolization after a significant learning curve: update on adverse events. J Personal Med. 2022;12(8):1261. https://doi.org/10.3390/jpm12081261.
Gil R, Shim DJ, Kim D, et al. Prostatic artery embolization for lower urinary tract symptoms via transradial versus transfemoral artery access: single-center technical outcomes. Korean J Radiol. 2022;23(5):548–54.
McWilliams JP, Bilhim TA, Carnevale FC, et al. society of interventional radiology multisociety consensus position statement on prostatic artery embolization for treatment of lower urinary tract symptoms attributed to benign prostatic hyperplasia: from the society of interventional radiology, the cardiovascular and interventional radiological society of Europe, societe francaise de radiologie, and the British society of interventional radiology: endorsed by the Asia Pacific society of cardiovascular and interventional radiology, Canadian association for interventional radiology, Chinese college of interventionalists, interventional radiology society of Australasia, Japanese society of interventional radiology, and Korean society of interventional radiology. J Vasc Int Radiol. 2019;30(5):627-637.e1.
Sandhu JS, Bixler BR, Dahm P, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA guideline amendment 2023. J Urol. 2024;211(1):11–9.
Acknowledgements
The PROstate Study Investigators include: Olivier Pellerin, MD, PhD, FSIR, FCIRSE (Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, France); Fabiane Barbosa, MD, PhD, MSc (Ospedale Niguarda Ca Granda, Milan, Italy); Peyman Borghei, MD (VA Long Beach Healthcare Systems, USA); Greg E Gin, MD (University of California, Irvine School of Medicine, USA; VA Long Beach Healthcare Systems, USA); Edward Uchio, MD, FACS, CPI (VA Long Beach Healthcare Systems, USA; UC Irvine Health, USA); Adam N Plotnik MD (Ronald Reagan UCLA Medical Center, USA); James H Briggs, FRCR (Royal Berkshire Hospital UK, United Kingdom); Andrew MacDonald, MD (Churchill Hospital UK, United Kingdom); Srini Tummala, MD, FSIR, FSVM (University of Miami- Miller School of Medicine, USA); Hemendra Navinchandra Shah, MD, MCh (University of Miami- Miller School of Medicine, USA); Issam M. Kably MD (University of Miami- Miller School of Medicine, USA); Keith Pereira, MD (Saint Louis University, USA); James Katrivesis, MD (UC Irvine Health, USA); Keng Lim Ng, MBBS, PhD, FRCS (Frimley Park Hospital, United Kingdom); Kirubahara Vaheesan, MD (Saint Louis University, USA); Mina Behdad, MSHCA (VA Long Beach Healthcare Systems, USA); Sarah MacGill, RN (Royal Berkshire Hospital UK, United Kingdom); Sarah Crosbie, RGN PGCert (Churchill Hospital UK, United Kingdom); Madita Gavrila, BSN (Churchill Hospital UK, United Kingdom); Susan Anthony, MD (Churchill Hospital UK, United Kingdom); Lia Quezada (University of Miami- Miller School of Medicine, USA); Ricardo Aleman, MBA (University of Miami- Miller School of Medicine, USA); Cynthia Toot Ferguson, ARNP (Holy Cross South Florida Medical Imaging, USA); Far Ahmed-Timms, RN (Royal Bournemouth and Christchurch Hospital, United Kingdom); Alexandra Edwards, CRN (Frimley Park Hospital) The authors wish to thank the patients and study monitors of this study. Analytical support in the preparation of this article was provided by Jerry Bounsanga, MSTAT, an employee of Merit Medical Systems, Inc. Medical writing and administrative support was provided by Gloria DeWalt, PhD, an employee of Merit Medical Systems, Inc.
Funding
This study was funded by Merit Medical Systems, Inc. Merit Medical Systems, Inc. was involved in the design and conduct of the study; data collection, management, analysis, and interpretation of the data; preparation and review of the manuscript; decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
MRS: conceptualization, investigation, writing—original draft, writing—review and editing; SB: conceptualization, investigation, writing—original draft, writing—review and editing; CD: investigation, writing—original draft, writing—review and editing; AR: investigation and writing—review and editing; FCC: writing—review and editing; CB: investigation, writing—review and editing; CRT: investigation, writing—review and editing; SB: investigation, writing—review and editing; JT: investigation, writing—review and editing; JSB: investigation, writing—review and editing; MR: investigation, writing—review and editing; JPM: conceptualization, investigation, writing—original draft, writing—review and editing; MWL: conceptualization, investigation, writing—original draft, writing–review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Marc R. Sapoval has received consulting fees from Merit Medical Systems, Inc.; Shivank Bhatia is a consultant and stock owner in Embolx, and has received research funding from Merit Medical Systems, Inc.; Carole Déan has received consulting fees from Merit Medical Systems, Inc.; Charles R. Tapping has received honoraria from Merit Medical Systems, Inc., and Boston Scientific is a consultant and proctor for Sirtex Medical; Justin P. McWilliams is a consultant for Asahi Intecc and Johnson & Johnson, lecturer for Penumbra Medical, Terumo Medical, and Siemens Medical; Mark W. Little is a consultant for Merit Medical Systems, Inc., Boston Scientific, Guerbet, Varian Medical, Crannmed, and Microbot; Antonio Rampoldi, Francisco César Carnevale, Clare Bent, Simone Bongiovanni, Jeremy Taylor, Jayson S. Brower, and Michael Rush have nothing to disclose.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Institutional Review Board Approval
Institutional review board approval was obtained from all participating centers prior to the initiation of the study. All patients provided written informed consent to participate in the study.
Previous Presentation
A synopsis of a portion of the study results was presented at the 2022 Global Embolization and Oncology Symposium Technologies (GEST) conference.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sapoval, M.R., Bhatia, S., Déan, C. et al. Two-Year Outcomes of Prostatic Artery Embolization for Symptomatic Benign Prostatic Hyperplasia: An International, Multicenter, Prospective Study. Cardiovasc Intervent Radiol (2024). https://doi.org/10.1007/s00270-024-03802-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00270-024-03802-0