Abstract
Purpose
The aim of PRISTINE was to evaluate the 6 and 12 months safety and efficacy of the Selution Sustained Limus Release (SLR)™ sirolimus-coated balloon for treatment of complex lower limb occlusive lesions (TASC II C & D) in patients with chronic limb threatening ischemia (CLTI) from Singapore.
Methods
PRISTINE was a prospective, non-randomized, single arm, observational, multi-investigator, single-center clinical study. Complication-free survival at 30 days was the safety clinical endpoint. Immediate technical success (ability to cross and dilate the lesion and achieve residual angiographic stenosis < 30%), 6-month primary vessel patency, limb salvage, clinically driven target lesion revascularization (TLR) and amputation free survival (AFS) were the efficacy endpoints of interest.
Results
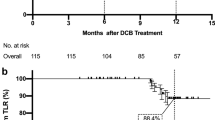
Seventy five patients were included. There were 50 (68.0%) males; mean age, 69.0 ± 10.7 years. CLTI severity was based on the Rutherford Scale (R5 = 51; R6 = 17). Significant co-morbidities included diabetes mellitus (n = 68; 91.0%) and end-stage renal failure (n = 28; 37.0%). 112 atherosclerotic lesions were treated (TASC II D = 58 (52%); 76 (67%) de novo). There was 100% technical success. Mean lesion length treated was 22.4 ± 13.9 cm. Primary vessel patencies at 6 and 12 months were 64/86 (74%) and 43/74 (58%) and freedom from clinically driven TLR were 72/86 (84%) and 55/74 (74%) respectively. AFS was 61/73 (84.0%; five deaths and seven major lower extremity amputation) at 6-months. Mean Rutherford score improved from 5.1 ± 0.55 at baseline to 1.1 ± 2.05 (p < 0.05) at one year and there was a wound healing rate of 38/48 (79%) at the same timepoint.
Conclusions
The Selution SLR™ drug eluting balloon is safe and efficacious in treating highly complex infra-inguinal atherosclerotic lesions in an otherwise challenging frail population of CLTI patients with a high incidence of diabetes and end-stage renal failure. It is associated with highly satisfactory acute technical and clinical success, 12-month target lesion patency and AFS.
Level of Evidence
Level 2b, Individual Cohort Study.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic limb threatening ischemia (CLTI) represents the most advanced stage of peripheral of artery disease (PAD), associated with high major amputation rates and cardiovascular mortality.[1] In recent years, technical options for endovascular revascularization have advanced considerably, leading some to advocate for an “endovascular-first” approach for all patients with CLTI.[2] However, existing evidence supports a selective revascularization algorithm, dependent on specific clinical and anatomic scenarios.[2] Patients with CLTI usually present with multi-level infra-inguinal PAD and tibial arterial occlusions.[3] Despite initial high rates of technical success (> 90%), plain balloon angioplasty is mired by high rates of restenosis because of elastic recoil[4] and barotrauma caused by the intra-arterial ballooning process, which leads to neointimal hyperplasia (NIH).[5] NIH increases reinterventions and delays wound healing in CLTI patients, increasing morbidity and mortality.[6]
Devices coated with rapamycin analogues such as sirolimus have been used successfully to limit restenosis by inhibiting the biologic pathway that leads to NIH.[7] The effects of rapamycin analogues have been well-studied in the coronary circulation, where sirolimus-eluting stents have been shown to be safe and more effective than paclitaxel devices.[8] In the peripheral arterial arena, the use of sirolimus in the form of sirolimus eluting balloons (SEBs), has only begun to gain traction in the past few years. The published data for the use of SEBs in the peripheral vasculature remains limited to a handful of small pilot studies up to 12 months.[9,10,11,12] Our group has previously reported promising outcomes at 6 and 12 months from the PRESTIGE study using the Selution Sustained Limus Release (SLR)™ (M. A. MedAlliance SA, Nyon, Switzerland) SEB, for the treatment of complex tibial occlusive lesions (TASC II C & D) in patients with CLTI from Singapore.[9, 11] We found a sustained low target lesion revascularization rate (TLR) of 7.4%, high amputation-free survival (AFS) (84%) and complete wound healing rates (82%) through to one year. However, the use of SEBs were limited to a relatively small CLTI cohort (n = 25), elution only to the below knee tibial arteries and contribution of patients were from mainly a single operator.
The aim of the PRISTINE registry was to extend the use of this SEB to both the femoro-popliteal segments and the below knee arteries in the CLTI setting, to include a larger number of patients and whether it could replicate the PRESTIGE results using multi-investigators from the same institution with varying peripheral vascular interventional experience.
Methods
PRISTINE Study Design
In the Prospective Registry to Investigate the Safety and efficacy of the Treatment with the SELUTION Sirolimus Drug Coated Balloon in TASC II C and D athero-occlusive Infra-iNguinal disease in patients with chronic limb threatening ischemia from SingaporE (PRISTINE) (NCT04534257), Selution SLR™ was studied for the treatment of complex femoro-popliteal and tibial artery occlusive disease with TASC II C and D lesions in CLTI patients from a multi-ethnic Asian cohort from Singapore.
The study was conducted in accordance with good clinical practice standards and the ethical principles of the Declaration of Helsinki and its amendments. The local Institutional Review Board approved this study. Pre-morbid variables collected prospectively included patient demographics, co-morbidities, tissue loss severity based on the Rutherford Scale[13] and pre-operative toe pressure. Procedural data included TASC II lesion severity,[14] number of superficial femoral, popliteal and tibial arteries treated. Lesion calcification was graded according to the presence of radio-opacities within the arterial wall at the site of occlusion or stenosis as per Dattilo.[15]
The study design, with inclusion and exclusion criteria are similar to the PRESTIGE trial, which has previously been described.[11] The target was to recruit a total of 75 patients with CLTI. All patients received the SEB according to the following protocol. The target lesion was mandatorily predilated with conventional balloon and angiography performed to confirm that less than 30% residual stenosis was successfully achieved in the target lesion. SEB was then deployed by balloon inflation to rated burst pressure of 10 atmospheres for 2 min to maximise drug transfer to the arterial wall, in a 1:1 sizing to the conventional balloon. The number of SEBs used to treat the target lesions were not restricted. If two devices were required to cover the lesion, the specified balloon overlap was at least 10 mm. For patients with multilevel disease, treatment with SEB included both the femoropopliteal and tibial lesions. Angioplasty balloon diameters of 2–7 mm and 150 mm lengths were available for PRISTINE.
Inclusion/Exclusion Criteria
Patients eligible for this study were ≥ 21 years of age with had a documented diagnosis of CLTI, presenting a score from 4 to 6 by Rutherford classification.[13] Angiographic inclusion criteria included de novo and post-PTA restenotic lesions located in the SFA/pop/tibial arteries suitable for endovascular therapy, target lesion length was > 50 mm and/or considered as TASC II C or D lesion according to the TASC II classification and the target lesion had angiographic evidence of stenosis > 50% or occlusion. Any patient with previous open vascular surgery of the index limb, undrained pus or spreading wet gangrene were excluded. The full eligibility criteria are as outlined in Table 1B.
Outcome Measures
Clinical primary safety outcome measure was freedom from major adverse clinical events (MACE), a composite of freedom from device- and procedure-related mortality through 30 days. Performance primary outcome measure was freedom from clinically driven TLR within 6 and 12 months post-index procedure. Clinically driven TLR was defined as any re-intervention performed for ≥ 50% diameter stenosis (on Duplex ultrasound) at the target lesion after documentation of recurrent or continuing clinical symptoms of the patient. Secondary outcome measures are as outlined in Table 1A.
Follow-up
Clinical assessments were performed at 1, 3 and 6 months to assess clinical progress and wound healing. At each follow-up, Walking Impairment Questionnaire (WIQ) and EuroQol-5 Dimension (EQ5D) was done to measure improvement in quality of life. There was a mandatory 6-month and 12-month Duplex ultrasound to check artery patency (Fig. 1).
Statistical Analysis
Baseline variables were summarised with the use of descriptive statistics. Continuous variables were reported as the mean and standard deviation, or median and range, as appropriate, and categorical variables as absolute number and percent. For TLR, amputation of the same limb or death were competing events that would preclude TLR, therefore the cumulative incidence of TLR was estimated from competing risks data using the cmprsk package in R. For AFS and death, the survival probability was estimated using Kaplan Meier analysis. All analyses were performed in R version 3.5.1.[16]
Results
Patient Demographics
Seventy five patients (54(72%) Chinese in origin) were enrolled over an 8-month period. 50/75 (66.7%) were male and mean age was 69.0 ± 10.7 years. 68/75(90.7%) had diabetes mellitus and 28/75 (37.3%) had end-stage renal failure (ESRF). 68/75 (90.7%) patients had some degree of tissue loss (Rutherford category 5 = 51/75(68.0%), Rutherford category 6 = 17/75(22.7%)) and mean presenting WIFI[17] score was 4.07 ± 1.43. Using the Society for Vascular Surgery clinical stages by expert consensus,[17] 51/75(68.0%) limbs were at high or moderate risk of major amputation at 1-year. Table 2 shows the subject demographics.
Lesion Characteristics
Seventy five legs (112 infrainguinal lesions; 76 de novo and 36 restenosis) were treated. 58/112(51.8%) were TASC D lesions. Mean lesion length treated was 22.4 ± 13.9 cm. The anterior tibial artery (ATA) was the most treated tibial vessel (28/112(25.0%)). There was a high incidence of moderate to severe vessel wall calcification (99/112(88.4%)). Majority of patients (48/75;64.0%) had multi-level atherosclerotic disease below the groin. Infra-malleolar disease was treated in (12/75,16.0%) limbs. Table 3 shows target lesion characteristics and procedural data.
Outcomes
There was 100% technical success. There were no retained device components events or device-related deaths. There was no balloon slippage during inflation. No device-related serious adverse events were reported during the first 30 days.
Target Lesion Primary Patency (TLPP) at 6 months was 64/86(74.4%) and freedom from clinically driven TLR was 72/86 (83.7%). AFS at 6 months was 61/73(83.6%) (five deaths and seven major lower extremity amputation). Two subjects were lost to follow-up. TLPP at 12 months was 43/74(58.1%) and the freedom from clinically driven TLR was 55/74(74.3%). AFS was 53/73(72.6%) (Table 4). Amputation-free survival probability and cumulative incidence of TLR and are presented in Figs. 2A, B respectively. The mean Rutherford score at baseline was 5.13 ± 0.55 and improved to 2.16 ± 2.42 and 1.09 ± 2.05 at 6 and 12 months respectively. There was a wound healing rate of 31/55(56.4%) and 38/48(79.2%) at 6 and 12 months respectively. 44/53(83.0%) patients showed improvement by at least 1 Rutherford category by 12 months (Fig. 3). There was a significant improvement in the EQ5D VAS Scores at 3, 6 and 12 months compared to baseline (Fig. 4) which may be related to wound healing and regaining independence to ambulate again.
Discussion
The PRISTINE registry is representative of everyday lower limb intervention practice for CLTI Asian patients in Singapore, who have long complex occlusive atherosclerotic lesions (mean of > 20 cm length) and heavy wall calcification. The data are relatively non-sanitized compared to RCTs, with liberal inclusion and few exclusion criteria. The strengths of the registry are that the follow-up of patients has been tight and prospective in nature with only few dropouts. The results demonstrated no peri-procedural early safety concerns, with no device complications or serious adverse events attributable to the device through to one year. It showed promising efficacy and clinical outcomes at 6 and 12 months, similar to other recent publications evaluating the performance of current SEBs on the market.[9, 11, 12] Despite high rates of diabetes mellitus, ESRF and moderate/severe vessel wall calcification, we have shown a 100% technical success rate (100%) and satisfactory 6- and 12- month primary vessel patencies of 74% and 58% respectively and freedom from clinically driven TLR of 84% (6 months) and 74% (1 year). An AFS of 84% and mortality rate of 7% at 6 months and 16% at 1 year are in keeping with what is expected from a challenging, frail population of patients with multiple co-morbidities.12-month primary patency rates for below-the-knee lesions and all-cause mortality rates were comparable to those reported in Stefanos et al.’s meta-analysis (59% vs 64% and 16% vs 9.0%, respectively). However, 12-month primary patency rates for above-the-knee lesions were lower in our cohort (57% vs 82.0%).[18] The SFA/pop subgroup is interesting in that the target lesion primary patency is lower than expected but fTLR is much higher. This may reflect SGH’s high threshold appetite for stenting an otherwise small Asian SFA/popliteal diseased artery because of concerns of stent complications.[19] The primary patency for these lesions were also lower compared to the XTOSI cohort of patients at both at 6 months (74% vs 88%) and 12 months (58% vs 79%).[12] XTOSI enrolled Asian CLTI patients with both fem-pop and BTK arterial lesions and used the MagicTouch PTA SEB (Concept Medical Inc., Florida, US), although their reporting definition was per patient rather than per lesion and the study included patients with intermittent claudication with less severe disease and co-morbidities on presentation. For BTK disease, the 6-month primary patency of 74% was comparable to MagicTouch.
A meta-analysis by Katsanos et al. reported significantly lower AFS in the paclitaxel-coated balloon arm as compared to plain balloon angioplasty, especially when high dose DCBs (3.0–3.5 μg/mm2) were used.[20] This was postulated to be due to distal non-target paclitaxel embolization resulting in micro-vessel occlusion, critical especially in the CLTI setting with minimal arterial reserve in the foot. One issue pertaining to the use of paclitaxel-based DCBs is the potential for the slow-flow phenomenon.[21] This was thought to be due to particulate embolization with the application of DCB with over 50% of drug lost down-stream reported.[22] This was thought to be a possible cause for the poorer outcomes with paclitaxel devices use reported in the meta-analyses by Katsanos et al.[23, 24] Early studies suggest no evidence of slow-flow phenomenon with the use of SEBs in the treatment of PAD, even for treatment of below ankle disease.[11] This was attributable to the micro-reservoirs of phospholipid polymer complex with the cell adherent technology of the Selution SLR™ SEB, which minimizes distal embolization.[21] Significant wound healing progress and improvement in ambulatory symptoms occurred with the balloon through 12 months. A complete wound healing rate of 79% is comparable, if not superior, with an open bypass salvage strategy [25] and previously published data on the use of paclitaxel coated balloons rates in lower limb endovascular revascularization for tissue loss.[26]
The results of PRISTINE may not be as impressive as those from PRESTIGE at the same time points, but this may be explained by the fact that PRESTIGE only included Rutherford 5 wounds whereas in PRISTINE, 22% had Rutherford 6 type advanced wounds and more than 50% of target lesions were TASC II D in nature. Furthermore, PRESTIGE only included tibial arterial lesions, whereas both femoro-popliteal and below the knee disease were allowed for PRISTINE, making the likelihood of mutli-level disease more likely to be treated with its intendent risks of restenosis and reintervention. While the initial results are encouraging and long-term follow-up is ongoing, the PRESTIGE Trial did not have a full range of SEB balloon sizes available to treat the entire vascular bed. PRISTINE included at least twice the number of patients to PRESTIGE, had the variable of multiple operators with differing peripheral vascular intervention experience using the technology and one of the aims was to determine whether complete revascularization of the affected vessel beds with SEBs will improve clinical outcomes as compared to treatment of only the tibial arteries. The results are encouraging in that they can reproduce similar results to PRESTIGE albeit with a bigger sample cohort with multiple proceduralists involved.
Limitations
PRISTINE’s limitations were its single arm, single centre, non-randomized nature and relatively small sample size with a self-adjudicated design. There was no comparator arm to draw to. No quantitative angiographic analysis was performed to look at exact lesion lengths, reference vessel diameters and luminal gain post angioplasty for each target lesion. Furthermore, there was no core lab adjudication for artery patency at follow-up and Duplex ultrasound was the modality of choice to assess TLPP, with its inherent limitation of operator bias. Furthermore, Duplex follow up was not core lab adjudicated. Whilst convenient, there is also no consensus to accurately determine vessel patency using Duplex ultrasound for BTK lesions.
Although this registry did not raise any early safety concerns, the data presented is through to one year only. Together with the small number of patients included, no conclusions can yet be made regarding the long term safety of SEBs. Another limitation was the attrition rate for primary patency assessments at 6 and 12 months; although mortality and major lower limb amputations were the main reasons for the drop out, which are expected for this type of frail cohort with multiple co-morbidities.
Furthermore, the wound care practiced at SGH for CLTI diabetic foot is relatively unique whereby the wound debridement and post-operative care are performed by the vascular specialist themselves in a timely manner with all the latest wound products and technology available to promote tissue granulation, which may limit the generalizability of our results.
Conclusions
Data from the PRISTINE registry show that the Selution SLR™ SEB is safe to use at least in the short term and efficacious in treating both above-the-knee and below-the-knee lesions, in a challenging cohort of Asian CLTI patients who have long complex occlusive atherosclerotic lesions and heavy wall calcification. It was associated with favorable 6 and 12-month primary patencies, freedom from clinical target lesion revascularization and foot wound-healing rates. Further studies are required to confirm these results in a larger cohort of patients in a RCT setting.
References
Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–8.
Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S-125S e40.
Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58:S1-S109 e33.
Baumann F, Fust J, Engelberger RP, et al. Early recoil after balloon angioplasty of tibial artery obstructions in patients with critical limb ischemia. J Endovasc Ther. 2014;21:44–51.
Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K-9K.
Sandra FBJR, Joana F, Celso C, João CS, Amílcar M. Neointimal Hyperplasia. Rev Port Cir Cardiotorac Vasc. 2019;26:213–7.
Marx SO, Marks AR. Bench to bedside: the development of rapamycin and its application to stent restenosis. Circulation. 2001;104:852–5.
Abizaid A. Sirolimus-eluting coronary stents: a review. Vasc Health Risk Manag. 2007;3:191–201.
Tang TY, Yap CJQ, Soon SXY, et al. 12-months results from the PRESTIGE study using sirolimus drug-eluting balloons in the treatment of complex btk tibial atherosclerotic lesions in CLTI patients. Cardiovasc Revasc Med. 2022;43:143–6.
Zeller T, Brechtel K, Meyer D-R, et al. Six-month outcomes from the first-in-human, single-arm SELUTION sustained-limus-release drug-eluting balloon trial in femoropopliteal lesions. J Endovasc Ther. 2020;27:683–90.
Tang TY, Yap C, Soon SXY, et al. World’s first experience treating TASC II C and D tibial occlusive disease using the selution SLR sirolimus-eluting balloon: six-month results from the PRESTIGE study. J Endovasc Ther. 2021;28:555–66.
Choke E, Tang TY, Peh E, et al. Magictouch PTA sirolimus coated balloon for femoropopliteal and below the knee disease: results from XTOSI pilot study up to 12 months. J Endovasc Ther. 2022;29:780–9.
Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38.
Norgren L, Hiatt WRJA, Dormandy, et al. Inter society consensus for the management of peripheral arterial disease. J Vasc Surg. 2007;45(1):S5–67.
Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360 degrees Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26:355–60.
RCT. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2018). URL: https://www.R-project.org/.
Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(220–34):e1-2.
Stefanos GSG, Sahil AP, Eric AS, Peter AS, Ehrin JA. Safety and efficacy of drug-coated balloon angioplasty for the treatment of chronic limb-threatening ischemia: a systematic review and meta-analysis. J Endovasc Ther. 2020;27:647–57.
Soon SXY, Patel A, Chong TT, et al. Distribution of peripheral arterial disease in patients undergoing endovascular revascularization for chronic limb threatening ischaemia: insights from the vascular quality initiative in Singapore. Vasc Specialist Int. 2021;37:13.
Katsanos KSS, Kitrou P, Krokidis M, Paraskevopoulos I, Karnabatidis D. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J Vasc Interv Radiol. 2020;31:202–12.
Tang TY, Sulaiman MSB, Soon SXY, et al. Slow-flow phenomena following lower limb paclitaxel- and sirolimus-coated balloon angioplasty in the setting of chronic limb threatening ischaemia-a case series. Quant Imaging Med Surg. 2022;12:2058–65.
Torii S, Jinnouchi H, Sakamoto A, et al. Comparison of biologic effect and particulate embolization after femoral artery treatment with three drug-coated balloons in healthy swine model. J Vasc Interv Radiol. 2019;30:103–9.
Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J Vasc Interv Radiol. 2020;31:202–12.
Katsanos K, Spiliopoulos S, Teichgraber U, et al. Risk of major amputation following application of paclitaxel coated balloons in thelower limb arteries: a systematic review and meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg 2022;63:60–71.
Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007;46:1180–90; discussion 1190.
Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–21.
Funding
The Manufacturer (M.A. MedAlliance SA) has provided an unrestricted research grant to defray costs of running the study and analysing the data.
M.A. MedAlliance SA, 001, Tjun Yip Tang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: TYT has received honoraria from M. A. MedAlliance, Biotronik, Philips, iVascular and Bard BD. SGH has received research and clinical trial study funds from Abbott Vascular, Bard BD, Biotronik, Boston Scientific, iVascular, M. A. MedAlliance and Medtronic.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, T.Y., Yap, C., Chan, S.L. et al. The Utility of Sirolimus Eluting Balloons in the Setting of Chronic Limb Threatening Ischaemia in Asian Patients from Singapore – 12 Months Results of the PRISTINE Registry. Cardiovasc Intervent Radiol 47, 863–874 (2024). https://doi.org/10.1007/s00270-024-03756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-024-03756-3