Abstract
Purpose
Transarterial radioembolization (TARE) with Yttrium-90 resin microspheres is a treatment option for patients with intrahepatic cholangiocarcinoma (ICC). However, optimising the timing of TARE in relation to systemic therapies and patient selection remains challenging. We report here on the effectiveness, safety, and prognostic factors associated with TARE for ICC in a combined analysis of the prospective observational CIRT studies (NCT02305459 and NCT03256994).
Methods
A combined analysis of 174 unresectable ICC patients enrolled between 2015 and 2020 was performed. Patient characteristics and treatment-related data were collected at baseline; adverse events and time-to-event data (overall survival [OS], progression-free survival [PFS] and hepatic PFS) were collected at every follow-up visit. Log-rank tests and a multivariable Cox proportional hazard model were used to identify prognostic factors.
Results
Patients receiving a first-line strategy of TARE in addition to any systemic treatment had a median OS and PFS of 32.5 months and 11.3 months. Patients selected for first-line TARE alone showed a median OS and PFS of 16.2 months and 7.4 months, whereas TARE as 2nd or further treatment-line resulted in a median OS and PFS of 12 and 9.3 months (p = 0.0028), and 5.1 and 3.5 months (p = 0.0012), respectively. Partition model dosimetry was an independent predictor for better OS (HR 0.59 [95% CI 0.37–0.94], p = 0.0259). No extrahepatic disease, no ascites, and < 6.1 months from diagnosis to treatment were independent predictors for longer PFS.
Conclusion
This combined analysis indicates that in unresectable ICC, TARE in combination with any systemic treatment is a promising treatment option.
Level of evidence: level 3, Prospective observational
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy after hepatocellular carcinoma (HCC). The incidence of ICC and ICC-related deaths are increasing, especially in Western countries, where tumours are associated with a dismal prognosis and short overall survival (OS) [1,2,3,4]. Complete surgical resection represents the only curative intent therapy [5,6,7]; however, 70% to 85% of ICC patients present with advanced disease where resection is no longer a treatment option [8,9,10,11].
First-line systemic therapy in patients with non-resectable tumours included gemcitabine or gemcitabine and cisplatin-based regimens, resulting in a median overall survival (OS) of 8.1 months and 11.7 months, respectively [12, 13] as a standard of care for many years. More recently, the administration of immune checkpoint inhibitors durvalumab or pembrolizumab in addition to a standard first-line combination chemotherapy resulted in a median OS likelihood of 12.8 and 12.7 months, respectively [14, 15]. For further line treatment, a European trial showed improvement in OS with the FOLFOX regimen compared to active symptom control (hazard ratio [HR] 0.69 [95% confidence interval [CI] 0.50–0.97, p = 0.031) [16], while a phase 3 trial in South Korea showed improved progression-free survival (PFS) when adding liposomal irinotecan to fluorouracil and leucovorin compared to fluorouracil and leucovorin alone (HR 0.56, 95% CI 0.39–0.81, p = 0.0019) [17]. However, resistance to systemic treatment is a known limitation in managing patients with ICC [18, 19].
Transarterial radioembolization (TARE) is an interventional therapeutic procedure that involves the targeted delivery of high doses of radiation to liver tumours via the hepatic artery. While robust evidence on the effectiveness of TARE in ICC is lacking, small population studies have suggested that TARE in the first-line palliative setting may provide additional benefits to the patients in light of other available systemic therapies [20]. Current guidelines indicate that patients with ICC may also benefit from receiving TARE as a second-line treatment after systemic therapy [5, 6].
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) initiated two prospective observational studies on the clinical application and outcomes of TARE with Yttrium-90 (Y90) resin microspheres (SIR-Spheres® Y90 resin microspheres, Sirtex Medical Pty Limited; St. Leonards, NSW, Australia): under the acronym CIRSE Registry for SIR-Spheres Therapy (CIRT), a Europe-wide cohort (NCT02305459) and a France-only cohort (NCT03256994) were collected. The present analysis combined the ICC cohorts collected in these CIRT studies to evaluate effectiveness outcomes after TARE in ICC and identify clinical characteristics as (potential) prognostic factors for effectiveness outcomes, to inform the optimal patient selection and treatment strategies.
Materials and Methods
Study Design
A pooled cohort of ICC patients from the Europe-wide and from the France-only studies were analysed. The CIRT studies are prospective, single-device, multi-centre observational studies with primary and metastatic hepatic malignancies treated with TARE using Y90 resin microspheres as the standard of care. The methodology of the CIRT study concept was published by Helmberger et al. [21]. For more insights on both cohorts, please refer to previously published papers [22,23,24,25].
In the European cohort, sites were invited to participate if they had a history of at least forty TARE cases, including ten cases within the twelve months prior to invitation. In the French cohort, all sites where TARE was performed were invited to participate regardless of prior experience with the treatment. Patient recruitment took place between January 2015 and December 2017, and between August 2017 and August 2020 in the CIRT and French CIRT studies, respectively. Follow-up data were collected until December 2019 in CIRT and until July 2022 in the French CIRT.
Data was collected using a customised electronic data capturing system and electronic case report form developed by ConexSys Inc. (Lincoln, RI, United States) and hosted on a local secure server in Vienna, Austria, maintained by ITEA (Vienna, Austria). Statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC, USA) and RStudio under R4.0.0 (R Foundation, Vienna, Austria).
Patient Selection
Patients included in the analysis were adults with histologically confirmed ICC and scheduled to receive TARE with Y90 resin microspheres. There were no specific exclusion criteria. All included patients signed an informed consent form. Procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
Participating sites were recommended to follow up with the patient every three months after the first TARE treatment. Due to the observational nature, actual follow-up intervals were left at the discretion of the investigators.
Assessments and Definitions
At the time of the first treatment, patient demographics, baseline data and treatment-related data were collected. Post-TARE treatments, safety data and time-to-event data were collected at every follow-up. Time-to-event was defined from the date of the first TARE treatment until the event date. Safety outcomes are described according to the Common Terminology Criteria for Adverse Events, version 4.03. Clinical parameters were disease status, tumour burden, procedures before and after TARE and dose methodology, as well as relevant blood markers including albumin, bilirubin, liver transaminases, International Normalised Ratio (INR) and Albumin-Bilirubin (ALBI) Grade (see Supplement 1 for the ALBI formula). Concomitant therapy was defined as the start of any systemic treatment with 56 days (8 weeks) before or after TARE.
Statistical Analysis
The datasets from both cohorts were combined and analysed. Since the case report forms and study proceedings were the same, no additional data manipulation was necessary. Data are presented as mean ± standard deviation or median (interquartile range [IQR]) for continuous variables and number (%) for categorical variables. Patients that died during the study were categorised as having progression for the PFS and hepatic PFS (hPFS) analysis.
The median OS, PFS and hPFS time were calculated with the associated 95% confidence interval using the Kaplan–Meier method and the median follow-up period was calculated using the reverse Kaplan–Meier method. A p-value of > 0.05 was considered statistically significant.
Multivariable survival analysis for OS, PFS and hPFS was performed using a Cox proportional-hazards model. The selection of variables was determined following a univariable analysis and a subsequent stepwise variable selection procedure with a significance level of 0.2 when deciding to enter a predictor into the stepwise model. The model with the lowest Akaike information criterion value was considered the final model. All available data were used, and no imputations of missing data were made. Missing data is indicated in the summary tables.
Results
Patient Demographics
One hundred seventy-four patients with ICC from 26 centres in eight European countries were included in this study, 120 patients from the European cohort and 54 from the French cohort (see Supplement 2). The median age was 64 (IQR 57–72), and 97/174 (55.7%) were male. The median time from diagnosis until first TARE was 6.1 months. Patients were in relatively good condition with Eastern Cooperative Oncology Group (ECOG) status mostly 0 (97, 55.7%) or 1 (61, 35.1%), and no extrahepatic disease in 124 (71.3%) of the patients (Table 1). Ascites and cirrhosis were observed in 13 (7.5%) and 21 (12.1%) of the patients, respectively. ALBI grade 1 was observed in 72 (41.4%) patients, grade 2 in 67 (38.5%) patients, and grade 3 in 1 (0.6%) patient. Baseline data from both cohorts separately are presented in Supplement 2.
Treatment Planning and Application
Bilobar tumours were found in 86 (49.4%) patients, and patients had one tumour (80, 46%), two to five tumours (35, 29.2%), six to nine tumours (11, 6.3%), ten or more tumours (12, 6.9%) or an uncountable number of tumours (36, 20.7%) (more details in Table 1). Tumour burden was < 10% (60, 35.3%), 10–20% (58, 34.1%) or > 20% (52, 31.6%). The prescribed activity was calculated using partition model dosimetry (50%) or body surface area (BSA) and modified BSA (50%). The median prescribed activity was 1.2 Giga-becquerel (GBq) (IQR 0.9–1.6) for whole liver treatments, 1.1 GBq (IQR 0.7–1.4) for right lobe treatments and 0.6 GBq (IQR 0.0–1.0) for left lobe treatments. The delivered activity was within 90% of the prescribed activity (i.e., technical success) in 170 (97.7%) cases.
The investigator-reported intention of TARE was primarily palliative (128, 73.6%) or downsizing (33, 19%) to potential curative treatment without pre-defined subsequent treatment (Table 2). Before TARE, 49 (28.2%) patients received locoregional treatments, mostly surgical resection (37, 75.5%) as opposed to transplantation. Sixty-two patients (35.6%) received TARE as monotherapy at first line, 20 (11.5%) received first-line TARE with concomitant systemic treatment, 54 patients (31%) had already received one line of systemic treatment, and 22 (12.6%) had received two or more lines of systemic treatment. After TARE, patients underwent additional locoregional treatments (16.7%) and/or systemic therapy sessions (48.3%).
Effectiveness
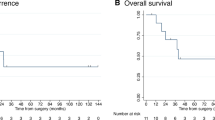
After a median follow-up of 26.2 months (per reverse Kaplan–Meier, 95% CI 24.4–28.5), 114 (65.5%) patients died, and 101 (58%) showed hepatic progression. The median OS for the entire cohort was 15.3 months (95% CI 11.2–19.1). Patients that received first-line TARE plus concomitant systemic treatment (20, 11.5%) had the longest median OS: 32.5 months (95% CI 11.8–37.0), patients receiving first-line TARE alone (62, 35.6%) had a median OS of 16.2 months (95% CI 9.0–27.7), patients receiving TARE as second line (with or without concomitant chemotherapy, 54, 31%) had a median OS of 12.0 months (95% CI 8.2–20.8), and at further lines (22, 12.6%) the median OS was 9.3 months (95% CI 4.5–14.7), p = 0.0028 (shown in Fig. 1A). A comparison between baseline characteristics between patients receiving first-line TARE plus concomitant systemic treatment and first-line TARE alone reveals no statistically significant differences between the cohorts (Supplement 3). The time from diagnosis to TARE per treatment line is reported in Supplement 4. Univariable analysis (Table 3) also showed favourable OS outcomes for patients with no extrahepatic disease or cirrhosis, partition model dosimetry and additional (locoregional and systemic) treatments after TARE. In the multivariable analysis (Table 4), partition model dosimetry (HR 0.59 [95% CI 0.37–0.94], p = 0.0259) was the only statistically significant independent prognostic factor for OS.
The median PFS was 6.0 months (95% CI 4.4–7.2), and the median hPFS was 6.4 months (95% CI 5.3–8.2). The position of TARE in the continuum of care (shown in Fig. 1B, C), no extrahepatic disease, and < 6.1 months from diagnosis until TARE predicted an improved PFS and hPFS, while < 20% tumour burden predicted an improved PFS (Table 3). In the multivariable analysis (Table 4), no extrahepatic disease, no ascites, and < 6.1 months from diagnosis to treatment were independent predictors for longer PFS (HR 0.64 [95% CI 0.44–0.92], p = 0.0177, HR 0.45 [95% CI 0.23–0.87], p = 0.0180, and HR 0.67 [0.47–0.97], p = 0.0350, respectively). For hPFS, independent predictors were no ascites (HR 0.51 [0.26–0.99], p = 0.0468) and TARE in the third line or beyond compared to first-line TARE (HR 1.90 [1.10–3.28], p = 0.0224). The complete univariable analyses for OS, PFS and hPFS can be found in Supplements 5–7.
Safety
During the study, 89 patients (51.1%) experienced at least one adverse event (Table 5), primarily mild (grade 1–2) adverse events such as abdominal pain (19.5%), fatigue (30.5%) or nausea (17.8%). Rarely, gastrointestinal ulcerations (3.4%), gastritis (1.1%) or REILD (2.3%) were observed. Severe adverse events (grade 3 and 4) were found in 28 (16.1%) patients: abdominal pain 5 (2.9%), fatigue 5 (2.9%), gastrointestinal ulceration 1 (0.5%), gastritis 1 (0.5%), radiation cholecystitis 1 (0.5%), REILD 3 (1.7%), and other 22 (12.6%). Supplement 9 shows the safety outcomes from the European and French cohorts separately.
Discussion
The present analysis results from the combined ICC cohorts of the prospective observational CIRT studies. Results indicate that patients receiving a combination of TARE with any systemic treatment as first-line treatment may have favourable OS, PFS and hPFS outcomes compared to TARE alone or to those receiving systemic therapy first. Moreover, further locoregional or systemic treatments after TARE are associated with favourable survival outcomes. Patients for whom dosimetry was determined using partition model dosimetry had longer OS outcomes than patients for whom dosimetry was determined by BSA or modified BSA. In terms of patient selection, patients without ascites and extrahepatic disease had better survival outcomes. Generally, safety results showed a good tolerability profile with 16.1% of the patients having reported any serious adverse events, 1.7% of these being REILD.
TARE and Systemic Therapy
The position of TARE in the treatment pathway of patients with ICC is contended. Our data show that patients treated with TARE plus concomitant systemic treatment had the best OS, PFS, and hPFS outcomes, compared to TARE alone or TARE after one or more lines of systemic treatments. However, the fact that these results did not maintain in the multivariable analysis may point towards a difference in patient presentation or other factors unaccounted for, which would go beyond the scope of this paper to explore in full. The benefits of TARE in treatment-naïve patients have been highlighted by other studies—albeit with smaller populations [26,27,28]. In 2017, Cucchetti et al. performed a meta-regression study and concluded that treatment-naïve patients with mass‐forming ICC should be selected as the best candidates with the possibility of adding concomitant standard systemic therapy [29]. In the phase 2 clinical trial published by Edeline et al., the authors concluded that a TARE plus concomitant systemic treatment (given one day before or after TARE) bears significant antitumor activity when used as first-line treatment, achieving an OS of 22 months [30]. At the same time, outcomes from the prospective observational RESiN study suggest that patients that received one line of systemic therapy had better outcomes after TARE compared to systemic therapy-naïve patients (19.1 vs 10.6 months, p = 0.07) [31], while a retrospective multicentre analysis of 128 ICC patients by Schaarschmidt et al. did not report any differences in survival related to treatment lines (p = 0.15) but did report an improved overall survival at any stage of treatment from first-line to salvage treatment due to the addition of TARE [32].
Recent randomised controlled trials have shown that the addition of immune checkpoint inhibitors durvalumab or pembrolizumab to standard first-line combination chemotherapy resulted in an improvement of OS likelihood of 1.3 months and 1.7 months, respectively, and is now considered as the standard of care [14, 15]. In our prospective observational setting, the patients that received first-line TARE plus any concomitant systemic treatment (within 56 days of TARE) had a median OS of 32.5 months. Despite the study design and potential confounding factors, TARE in combination with systemic treatment could be a promising first-line treatment in patients with unresectable ICC. Further research into the molecular structures of ICC suggests that patients with fibroblast growth factor receptor (FGFR)-2 fusions respond better to second-line therapies [33, 34], potentially complicating the results of systemic treatments in ICC. In the meantime, the outcome of this prospectively collected real-world dataset provides further insight into the optimal place of TARE in the treatment pathway of patients with ICC.
Prognostic Factors
The multivariable analysis identified partition model dosimetry as an independent prognostic factor for an improved OS. Evidence from the recent Phase 2 DOSISPHERE-01 trial showed that personalised dosimetry methods such as partition model dosimetry in glass microspheres could improve survival outcomes in patients with HCC, compared to standard dosimetry [35, 36], which was recently confirmed in resin microspheres by a large prospective observational cohort [37]. Based on the evidence in HCC, an international expert group recommended using partition model or voxel-based dosimetry for activity prescription in resin microspheres when either whole liver or selective, non-ablative or ablative TARE is planned, with a mean absorbed dose to non-tumoural liver of 40 Gy and minimum mean tumour-absorbed dose of 100–120 Gy [38]. Indeed, a small prospective study with 38 ICC patients suggested that for patients receiving resin microspheres, a mean tumour dose of ≥ 75 Gy or a maximum tumour dose of ≥ 150 Gy was associated with a median OS of 20.2 months compared to 6.5 months for those receiving less (p = 0.001 and 0.002, respectively) [39]. This prospective cohort confirms that real-world patients with ICC may benefit from personalised dosimetry compared to standard dose calculation models (BSA and modified BSA). Our study was not designed with detailed dosimetry-related outcomes in mind, and further research into this topic should be considered.
Several systematic reviews and meta-analyses on studies on TARE in ICC found similar OS results as this study but underlined the significant heterogeneity of treated patients in the retrieved studies [20, 40,41,42]. Our results emphasise the observations from previous studies that a local treatment such as TARE should not be the treatment of choice in ICC patients with extra-hepatic disease, ascites, or extensive tumour burden [27, 30, 43,44,45,46,47,48,49,50]. Other studies have identified prior treatments [47, 51] and tumour response [27, 45, 50, 52] as other significant prognostic factors. Köhler et al. found that the extent of liver disease to one or both liver lobes was associated with survival, irrespective of tumour volume (p = 0.041) [51].
Limitations
Limitations of this prospective observational study are the existence of potentially critical confounding factors, which could not be controlled. The heterogeneity of the patient population reflects the real-life clinical practice in participating sites and, thus, its diversity in patient selection and clinical outcomes. We used a multivariable analysis to alleviate, to some degree, this heterogeneity but potential confounding factors not included in the analysis should be considered when interpreting the results. It is essential to consider that the patients in the European cohort were recruited between 2015 and 2017, while the patients in the French cohort were recruited between 2017 and 2019. Any potential changes in practice over time, for example changes in the delivery systems, imaging models allowing for more precise tumour targeting and better patient selection were not considered when analysing the results but should be considered when interpreting them.
The relatively high number of censored patients for the analysis of OS (60, 34.5%) and PFS (27, 15.5%) is comparable to other studies in oncology [53]. Investigators’ reports confirmed that TARE requires a comprehensive hospital infrastructure, leading to referrals from physicians who follow up with the patient after the treatment. Follow-up information was, in those cases, obtained by contacting the referring physician or, if this was not possible, the patient was considered as lost to follow-up. Moreover, interventional radiology departments did not always have the appropriate infrastructure to perform follow-ups consistently, contributing to the increased number of censored patients during follow-ups. Finally, central tumour response assessment was not done. Instead, response assessment was performed by the sites with various criteria (e.g., Response Evaluation Criteria in Solid Tumours (RECIST), modified RECIST or Positron Emission Tomography Response Criteria in Solid Tumours (PERCIST)) according to local habits and expertise of centres. This prevented us from including tumour response in the analysis.
Conclusion
This analysis represents a large prospective cohort of patients with nonresectable ICC treated with TARE. Despite the limitations of a real-world study, the results suggest that TARE combined with concomitant systemic treatment could be considered as an early treatment modality in the treatment pathway of patients with liver-only ICC, also considering its low toxicity. Our findings suggest the need for more studies to account for further confounders and to be able to draw confident conclusions about these combination treatments.
References
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80.
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62.
Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–73; discussion 73–5.
Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104–14.
Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v28–37.
Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(5):541–65.
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88.
Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58(7):1048–55.
Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221(6):1041–9.
Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Soreide K, Wigmore SJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 2021;273(2):240–50.
van Vugt JLA, Gaspersz MP, Coelen RJS, Vugts J, Labeur TA, de Jonge J, et al. The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB (Oxford). 2018;20(1):83–92.
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–74.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–65.
Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence. 2022;1(8).
Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701.
Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560–72.
Marin JJG, Lozano E, Briz O, Al-Abdulla R, Serrano MA, Macias RIR. Molecular bases of chemoresistance in cholangiocarcinoma. Curr Drug Targets. 2017;18(8):889–900.
Marin JJG, Lozano E, Herraez E, Asensio M, Di Giacomo S, Romero MR, et al. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1444–53.
Edeline J, Lamarca A, McNamara MG, Jacobs T, Hubner RA, Palmer D, et al. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: a systematic review and pooled analysis. Cancer Treat Rev. 2021;99: 102258.
Helmberger T, Arnold D, Bilbao JI, de Jong N, Maleux G, Nordlund A, et al. Clinical application of radioembolization in hepatic malignancies: protocol for a prospective multicenter observational study. JMIR Res Protoc. 2020;9(4): e16296.
Helmberger T, Golfieri R, Pech M, Pfammatter T, Arnold D, Cianni R, et al. Clinical application of trans-arterial radioembolization in hepatic malignancies in Europe: first results from the prospective multicentre observational study CIRSE registry for sir-spheres therapy (CIRT). Cardiovasc Intervent Radiol. 2021;44:21–35.
Loffroy R, Ronot M, Greget M, Bouvier A, Mastier C, Sengel C, et al. Short-term safety and quality of life outcomes following radioembolization in primary and secondary liver tumours: a multi-centre analysis of 200 patients in France. Cardiovasc Intervent Radiol. 2021;44:36–49.
Schaefer N, Grözinger G, Pech M, Pfammatter T, Soydal C, Arnold D, et al. Prognostic factors for effectiveness outcomes after transarterial radioembolization in metastatic colorectal cancer: results from the multicentre observational study CIRT. Clin Colorectal Cancer. 2022;21:285–96.
Maleux G, Albrecht T, Arnold D, Bargellini I, Cianni R, Helmberger T, et al. Predictive factors for adverse event outcomes after transarterial radioembolization with yttrium-90 resin microspheres in Europe: results from the prospective observational CIRT study. Cardiovasc Intervent Radiol. 2023;46:852–67.
Edeline J, Du FL, Rayar M, Rolland Y, Beuzit L, Boudjema K, et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med. 2015;40(11):851–5.
Mosconi C, Gramenzi A, Ascanio S, Cappelli A, Renzulli M, Pettinato C, et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer. 2016;115(3):297–302.
Sarwar A, Ali A, Ljuboja D, Weinstein JL, Shenoy-Bhangle AS, Nasser IA, et al. Neoadjuvant Yttrium-90 transarterial radioembolization with resin microspheres prescribed using the medical internal radiation dose model for intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2021;32(11):1560–8.
Cucchetti A, Cappelli A, Mosconi C, Zhong JH, Cescon M, Pinna AD, et al. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: a meta-regression study. Liver Int. 2017;37(7):1056–64.
Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):51–9.
Robinson TJ, Du L, Matsuoka L, Sze DY, Kennedy AS, Gandhi RT, et al. Survival and toxicities after yttrium-90 transarterial radioembolization of cholangiocarcinoma in the RESiN registry. J Vasc Interv Radiol. 2022;34(4):694–701.e3.
Schaarschmidt BM, Kloeckner R, Dertnig T, Demircioglu A, Muller L, Auer TA, et al. Real-life experience in the treatment of intrahepatic cholangiocarcinoma by (90)Y radioembolization: a multicenter retrospective study. J Nucl Med. 2022;64(4):529–35.
Abou-Alfa GK, Bibeau K, Schultz N, Yaqubie A, Millang B, Ren H, et al. Effect of FGFR2 alterations on overall and progression-free survival in patients receiving systemic therapy for intrahepatic cholangiocarcinoma. Target Oncol. 2022;17(5):517–27.
Bibeau K, Feliz L, Lihou CF, Ren H, Abou-Alfa GK. Progression-free survival in patients with cholangiocarcinoma with or without FGF/FGFR alterations: a FIGHT-202 post hoc analysis of prior systemic therapy response. JCO Precis Oncol. 2022;6: e2100414.
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29.
Garin E, Tzelikas L, Guiu B, Chalaye J, Edeline J, Baere TD, et al. Major impact of personalized dosimetry using 90Y loaded glass microspheres SIRT in HCC: Final overall survival analysis of a multicenter randomized phase II study (DOSISPHERE-01). J Clin Oncol. 2020;38(4_suppl):516.
Kolligs F, Arnold D, Golfieri R, Pech M, Peynircioglu B, Pfammatter T, et al. Factors impacting survival after transarterial radioembolization in patients with hepatocellular carcinoma: results from the prospective CIRT study. JHEP Rep. 2023;5(2): 100633.
Levillain H, Bagni O, Deroose CM, Dieudonne A, Gnesin S, Grosser OS, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48(5):1570–84.
Cheng B, Sethi I, Davisson N, Brandon D, Barron B, Galt J, et al. Yttrium-90 dosimetry and implications on tumour response and survival after radioembolisation of chemo-refractory hepatic metastases from breast cancer. Nucl Med Commun. 2021;42(4):402–9.
Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, et al. Transarterial chemoembolization and radioembolization for unresectable intrahepatic cholangiocarcinoma-a systemic review and meta-analysis. Cardiovasc Intervent Radiol. 2021;44(5):728–38.
Schartz DA, Porter M, Schartz E, Kallas J, Gupta A, Butani D, et al. Transarterial yttrium-90 radioembolization for unresectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol. 2022;33(6):679–86.
Yu Q, Liu C, Pillai A, Ahmed O. Twenty years of radiation therapy of unresectable intrahepatic cholangiocarinoma: internal or external? A systematic review and meta-analysis. Liver Cancer. 2021;10(5):433–50.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29(8):1101–8.
Hoffmann RT, Paprottka PM, Schon A, Bamberg F, Haug A, Durr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–16.
Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24(8):1227–34.
Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–8.
Reimer P, Virarkar MK, Binnenhei M, Justinger M, Schon MR, Tatsch K. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of yttrium-90 radioembolization: results in therapy-naive patients. Cardiovasc Intervent Radiol. 2018;41(5):744–52.
White J, Carolan-Rees G, Dale M, Patrick HE, See TC, Bell JK, et al. Yttrium-90 transarterial radioembolization for chemotherapy-refractory intrahepatic cholangiocarcinoma: a prospective, observational study. J Vasc Interv Radiol. 2019;30(8):1185–92.
Bargellini I, Mosconi C, Pizzi G, Lorenzoni G, Vivaldi C, Cappelli A, et al. Yttrium-90 radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Intervent Radiol. 2020;43(9):1305–14.
Kohler M, Harders F, Lohofer F, Paprottka PM, Schaarschmidt BM, Theysohn J, et al. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with yttrium-90 radioembolization. J Clin Med. 2019;9(1):56.
Swinburne NC, Biederman DM, Besa C, Tabori NE, Fischman AM, Patel RS, et al. Radioembolization for unresectable intrahepatic cholangiocarcinoma: review of safety, response evaluation criteria in solid tumors 1.1 imaging response and survival. Cancer Biother Radiopharm. 2017;32(5):161–8.
Dudley WN, Wickham R, Coombs N. An Introduction to Survival Statistics: Kaplan-Meier Analysis. J Adv Pract Oncol. 2016;7(1):91–100.
Acknowledgements
The authors want to thank the patients, the investigators, and the site staff involved in the studies, particularly the local study nurses that contributed significantly to the quality of the collected data through feedback and comments during the data collection phase. The authors are indebted to Prof. José Ignacio Bilbao, who initiated the study and was Chairperson of the CIRT Steering Committee between 2015 and 2018 and is now enjoying his well-deserved retirement. CIRSE, the Cardiovascular and Interventional Radiological Society of Europe, is responsible for the independent execution of the CIRT studies and has sole ownership of the data. Statistical analysis for the European cohort and the combined cohort was performed by independent statistician Helena Pereira, Paris, France. Statistical analysis for the French cohort was performed by OPIS, Desio, Italy. The electronic data capturing system was developed and supported by ConexSys Inc (Lincoln, RI, United States), and a customised data management system was designed by Joaquin Padilla Montani (Vienna, Austria). ITEA GmbH (Vienna, Austria) developed and maintained the necessary server infrastructure. The authors thank the CIRSE Central Office and the CIRSE Clinical Research department staff, specifically Agnes Walk, for their support during the design, setup, and management of the study and drafting of the manuscript.
On behalf of the European Investigators: Thomas Albrecht, Vivantes Klinikum Neukölln, Department for Radiology and Interventional Therapy, Berlin, Germany; Olivier D’Archambeau, University Hospital Antwerp, Department of Radiology, Antwerp, Belgium; Tugsan Balli, Cukurova University, Radiology Department, Balcalı Hospital, Adana, Turkey; Sadik Bilgic, Ankara University, Department of Radiology, Medical Faculty, Ankara, Turkey; Allan Bloom, Hadassah-Hebrew University Medical Center, Department of Radiology, Jerusalem, Israel. Roberto Cianni, Department of Interventional Radiology, S. Camillo Hospitals, Rome, Italy. Roberto Cioni, University of Pisa, Diagnostic and Interventional Radiology, Department of Translational Research and New Technologies in Medicine, Pisa, Italy. Roman Fischbach, Asklepios Klinik Altona, Department of Radiology and Neuroradiology, Hamburg, Germany. Patrick Flamen, Institute Jules Bordet, Université Libre de Bruxelles, Nuclear Medicine Department, Brussels, Belgium. Laurent Gerard, University Hospital of Liege, Division of Radiology, Liège, Belgium; Rita Golfieri, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Italy; Gerd Grözinger, Eberhard Karls University, Department of Diagnostic and Interventional Radiology, Tübingen, Germany; Thomas Helmberger, Department of Radiology, Neuroradiology and Minimal-Invasive Therapy, Klinikum Bogenhausen, Munich, Germany. Marcus Katoh, Helios Hospital Krefeld, Department of Diagnostic and Interventional Radiology, Krefeld, Germany. Michael Koehler, University Hospital Muenster, Department of Clinical Radiology, Muenster, Germany; Jan Robert Kröger, Johannes Wesling Klinik Minden, Universitätsinstitut für Radiologie, Neuroradiologie und Nuklearmedizin der Mühlenkreiskliniken, Minden, Germany; Christiane Kuhl, University Hospital Aachen, Department of Radiology, Aachen, Germany; Geert Maleux, Radiology, Univeritair Ziekenhuis Leuven, Leuven, Belgium; Franco Orsi, European Institute of Oncology, Interventional Radiology Division, Milan, Italy. Murat Özgün, St. Franziskus Hospital, Department of Radiology, Muenster, Germany; Maciej Pech, Department of Radiology and Nuclear Medicine, University of Magdeburg, Magdeburg, Germany; Thomas Pfammatter, Institute of Diagnostic and Interventional Radiology, Universitätsspital Zürich, Zürich, Switzerland; Peter Reimer, Academic Teaching Hospital the University of Freiburg, Städtisches Klinikum Karlsruhe, Institute for Diagnostic and Interventional Radiology, Karlsruhe, Germany; Maxime Ronot, APHP, University Hospitals Paris Nord Val de Seine, Department of Radiology, Beaujon, Clichy, Hauts-de-Seine, France; Bruno Sangro, Liver Unit and HPB Oncology Area, Clínica Universidad de Navarra and CIBEREHD, Pamplona, Spain; Axel Schmid, University Hospital Erlangen, Department of Radiology, Erlangen, Germany; Alessandro Vit, Azienda Ospedaliero Universitaria, SOC Diagnostica Angiografica e Radiologia Interventistica, Udine, Italy.
On behalf of the French Investigators: Charles Mastier, Centre Léon Bérard, Interventional Radiology, Lyon, France; Antoine Bouvier, CHU d‘Angers, Department of Radiology, Angers, France; Romaric Loffroy, CHU de Dijon, Department of Vascular and Interventional Radiology, Image-Guided Therapy Center, Dijon, France; Maxime Ronot, Université Paris Cité, Paris, CRI, INSERM 1149 & Department of Radiology, Hôpital Beaujon APHP Nord, Clichy, France; Michel Greget, Service d’Imagerie interventionnelle, Hôpital de Hautepierre—CHRU de Strasbourg, Strasbourg, France; Lambros Tselikas, Institut Gustave Roussy, Interventional Radiology, Département d’anesthésie Chirurgie et Interventionel (DACI), Paris, France; Christian Sengel, CHU de Grenoble Alpes, Interventional Radiology, Grenoble, France; Gilles Piana, Institut Paoli-Calmettes, Interventional Radiology, Marseille, France; Julien Frandon, CHU de Nimes, Interventional Radiology, Nimes, France; Jean-Pierre Tasu, CHU de Poitiers, Interventional Radiology, Poitiers, France; Hicham Kobeiter, Hôpital Henri Mondor, Interventional Radiology, Paris, France; Jean Baptiste Pinaquy, Hôpital Haut Lévêque—CHU de Bordeaux, Bordeaux, France; Pierre-Jean Valette, Hôpital Edouard Herriot, Department of Radiology, Lyon, France; Olivier Pellerin, Hôpital Européen Georges Pompidou, Interventional Radiology, Paris, France.
Funding
The CIRT studies (NCT02305459 and NCT03256994) were funded by an independent investigator-initiated research grant from SIRTEX Medical Europe GmbH (Bonn, Germany). CIRSE, the Cardiovascular and Interventional Radiological Society of Europe, is responsible for the independent execution of the CIRT studies and has sole ownership of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Valérie Vilgrain recieved payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and payment for expert testimony from Sirtex. Dirk Arnold received consulting fees and honoraria for presentations and lectures and travel support from Boston Scientific and Terumo, MSD, BMS, AstraZeneca, Roche, Servier, Sanofi and Merck Serono. He is on the guidelines committee of the European Society for Medical Oncology, and supported oncopolicy manuscripts for the European Cancer Organisation. Maxime Ronot received honoraria for lectures from GE Healthcare, Ipsen, Canon-Toshiba, Alexion Pharmaceuticals, Guerbet, and Sirtex. Geert Maleux received honoraria for speaker’s bureau from Sirtex Medical and operated as a proctor for Sirtex. Bruno Sangro received grants or contracts from Sirtex and BMS, consulting fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca, Bayer, BMS, Eisai, Incyte, Ipsen, Roche, Sirtex Medical; Participation on a Data Safety Monitoring Board or Advisory Board from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo, and has a leadership or fiduciary role in the International Liver Cancer Association. Peter Reimer, Tugsan Balli, Rita Golfieri, Romaric Loffroy, Cristina Mosconi, Christian Sengel, Niklaus Schaefer, Graham Munneke, Bora Peynircioglu, Nathalie Kaufmann, Maria Urdaniz, Helena Pereira, Niels de Jong and Thomas Helmberger had nothing to declare.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study has been approved by applicable ethics committees. All participants signed an informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reimer, P., Vilgrain, V., Arnold, D. et al. Factors Impacting Survival After Transarterial Radioembolization in Patients with Unresectable Intrahepatic Cholangiocarcinoma: A Combined Analysis of the Prospective CIRT Studies. Cardiovasc Intervent Radiol 47, 310–324 (2024). https://doi.org/10.1007/s00270-023-03657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03657-x