Abstract
Purpose
To evaluate the use of drug-coated balloons in a real-world patient population with peripheral arterial disease and analyse the impact of sex on mid-term outcomes following their utilisation.
Methods
The BIOLUX P-III is a prospective, international, multi-centre, registry of patients with infra-inguinal lesions treated using the Passeo-18 Lux, a drug-coated balloon. Our study is a 24-month subgroup analysis of these patients; primary endpoints were freedom from major adverse events and clinically driven target lesion re-vascularisation within 12 months post-intervention.
Results
Of the 877 patients in the registry, 561 (64.0%) were male and 316 (36.0%) were female. Chronic limb threatening ischaemia (Rutherford class ≥ 4) occurred in 35.7% of males and 40.6% of females. Rates of freedom from major adverse events and clinically driven target lesion re-vascularisation at 12 months were 87.3% (95% confidence interval [CI] 84.2–89.9) and 90.4% (95% CI 86.5–93.3), and 92.3% (95% CI 89.9–94.1) and 92.9% (95% CI 89.7–95.1) in males and females, respectively. All-cause mortality at 24 months was 12.0% (95% CI 9.4–15.3) in males and 11.9% (95% CI 8.6–16.5) in females. The major target limb amputation rate at 24 months was 9.1% (95% CI 6.9–11.9) in males and 4.0% (95% CI 2.3–7.0) in females.
Conclusion
Treatment with the Passeo-18 Lux DCB demonstrated high efficacy and low complication rates. Despite the greater proportion of chronic limb threatening ischaemia observed in females, males were at a greater risk of ipsilateral major limb amputation and major adverse events following drug-coated balloon utilisation.
Clinical Trial Registration
NCT02276313.
Level of Evidence
Level 4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of peripheral arterial disease (PAD) is increasing [1]; however, its recognition remains lacking. While the total population burden of PAD appears to be higher in females, they are currently under-represented within prospective studies [2], leading to a paucity of sex-directed management strategies for PAD. Whether sex-related differences in presentation—such as smaller vessel diameters and increased likelihood of tibial occlusive disease in females—should be associated with different endovascular treatment modalities (i.e. balloon angioplasty vs. stenting) remains unknown [3, 4].
The role of drug-coated balloons (DCBs), such as the Passeo-18 Lux DCB (Biotronik, Berlin, Germany), has previously been outlined in trials such as the BIOLUX P-I and P-II [5,6,7]. Still, despite demonstrating superiority in treating symptomatic femoropopliteal lesions, there remains a concern regarding their effect in females [3]. There has been an under-representation of females in studies while they have lacked a specific gender-based endpoint. The majority of data is obtained from post hoc analysis, such as the LEVANT II study, which failed to identify a treatment effect in females [8]. However, a recent follow-up of the PTA THUNDER trial highlighted that the target lesion re-vascularisation (TLR) rate in females treated with DCBs was twice that of males [9]. Despite higher reintervention rates in females, a recent study has highlighted that males with PAD have an increased risk of mortality and major adverse cardiovascular events (MACEs) following endovascular re-vascularisation [10].
Given the previously identified differences in both presentation and outcomes following re-vascularisation, we aimed to study sex differences following DCB (the Passeo-18 Lux) utilisation in the BIOLUX P-III registry and its impact on the TLR and re-stenosis or occlusion rates. Patient data were previously published [11,12,13].
Methods
Study Design and Population
The BIOLUX P-III is a prospective, international, all-comers registry—including 44 centres across Asia, Australia, and Europe—that aimed to confirm the safety and effectiveness of the Passeo-18 Lux DCB for atherosclerotic disease of the infra-inguinal arteries; 877 patients were included. The study design was previously reported [11,12,13]; adults with infra-inguinal lesions suitable for endovascular therapy with the Passeo-18 Lux DCB were included. Exclusion criteria were a life expectancy of < 1 year, participation in another clinical trial, pregnancy, and failure to successfully cross the target lesion with a guidewire. Follow-up was initially planned for 24 months; however, due to the controversy regarding elevated mortality rates associated with the use of paclitaxel [14, 15], follow-up was extended to 5 years to collect mortality data.
The study was conducted according to the principles of the Declaration of Helsinki (ISO14155:2011) and local regulations and was approved by the respective independent ethics committees. All patients provided written informed consent. Endpoint-related data were monitored in at least 25% of patients. A clinical events committee adjudicated all major adverse events (MAEs), TLRs, and deaths. The trial is registered at ClinicalTrials.gov: NCT02276313.
Study Device
The Passeo-18 Lux DCB (diameter: 2.0–7.0 mm, length: 40–120 mm) is built on the base of the Passeo-18 percutaneous transluminal angioplasty catheter. Incorporation of paclitaxel into the delivery matrix of Butyryl-tri-hexyl-citrate binds the drug into a microcrystalline structure, improving vessel wall infiltration. A sheath protects the balloon, maintaining its profile and drug coating, and is used as an insertion aid when the catheter is advanced through the introducer sheath. The DCB was used following the manufacturer’s instructions according to standard clinical practice.
Definitions and Outcome Measure
Technical success was defined as successful completion of the endovascular procedure combined with immediate morphological success (≤ 50% reduction in residual diameter of the treated lesion), determined using visual estimation. Device success was defined as the successful delivery, inflation, deflation, and retrieval of the DCB; procedural success was defined as technical and device success without any MAE during hospital stay. Primary patency was defined as freedom from CD-TLR or restenosis, determined using a duplex ultrasound (not mandated for all patients) peak systolic velocity ratio of ≤ 2.5. MAEs were defined as device- and procedure-related mortality within 30 days, and major target limb amputation and clinically driven TLR (CD-TLR) at 6, 12, and 24 months post-index procedure. CD-TLR was defined as reintervention for stenosis (> 50% diameter) after documenting recurrent clinical symptoms.
The primary outcomes measures of our analysis, compared between sexes, were freedom from MAEs and CD-TLR within 12 months of intervention. The secondary outcome measures were technical and procedural success; freedom from MAEs and CD-TLR at 6 and 24 months; clinically driven target vessel re-vascularisation (CD-TVR); minor and major target limb amputation (any amputation above the ankle); and change in ankle-brachial index (ABI), Rutherford classification, and patient-reported outcomes (pain scale and walking impairment questionnaire) at 6, 12, and 24 months. The primary patency was assessed at 12 and 24 months.
Statistical Analyses
Hypothesis-driven sample size estimation was not performed. For continuous variables, descriptive statistics included mean ± standard deviations and range; for categorical variables, the absolute and relative frequencies were calculated. Two-sided 95% confidence intervals (CIs) were calculated when appropriate. Freedom from CD-TLRs and MAEs and their individual components were estimated using Kaplan–Meier analysis, and intergroup comparisons were performed with the log-rank test. Standard errors were calculated using the Greenwood formula. Estimates were presented with 95% CIs. The Chi-squared or Fisher’s exact test was used to compare categorical or binary variables, while the Student’s t-test or Wilcoxon rank-sum test was utilised for continuous variables of independent samples. For ordinal data, the Cochran-Armitage test for trend was used. Follow-up comparisons were performed using the Wilcoxon signed-rank test. Statistical significance was set at p < 0.05; statistical calculations were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Of the 877 patients in the registry, 561 (689 lesions) were male and 316 (395 lesions) were female (Fig. 1). Concomitant co-morbidities such as coronary artery disease (46.7% vs. 33.5%, p < 0.001) and diabetes mellitus (51.3% vs. 41.1%, p = 0.004) were more common in males. Conversely, hypertension (83.4% vs. 87.3%, p = 0.120) was more common in females; however, this lacked statistical significance (Table 1). Chronic limb threatening ischaemia (CLTI; Rutherford class ≥ 4) was present in 35.7% of males and 40.6% of females. Additionally, females were older than males during intervention and were more likely to be active smokers.
The superficial femoral artery was the most frequent location for lesions in males and females (54.0% vs. 54.9%), followed by the popliteal segment (18.7% vs. 25.3%) (Table 2); most lesions were de novo (55.3% vs. 51.9%). Total occlusions (22.9% vs. 28.4%) and in-stent restenosis (10.4% vs. 11.1%) were more common in females. Most lesions in both groups were calcified, while 17.6% and 11.6% comprised heavy calcification. The reference vessel diameter (RVD) was smaller in females (4.7 ± 1.2 mm. vs. 4.5 ± 0.9 mm., p < 0.001), who also demonstrated more Transatlantic Inter-Society Consensus (TASC) D lesions, lesions > 200 mm in length, and active ulcerations during intervention than males.
The device was successfully deployed in 99.8% of cases, with few lesions in both groups requiring adjunctive bailout procedures after DCB application with a stent (16.8% vs. 13.7%) (Table 3). The clinical events committee (CEC) adjudicated rates of freedom from MAEs in males and females of 91.7% and 95.1% at 6 months; 87.3% and 90.4% at 12 months; and 81.2% and 86.4% at 24 months, respectively (p = 0.066) (Fig. 2). Six, 12, and 24 months post-procedure, 95.9%, 92.3%, and 87.3% of males, and 96.6%, 92.9% and 89.3% of females remained free from CD-TLR, respectively (p = 0.408) (Fig. 3).
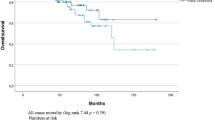
All-cause mortality at 24 months was 12.0% in males and 11.9% in females (Fig. 4). Death was most common between 12 and 24 months post-procedure for both sexes; however, this was not significant (p = 0.956). Cardiac-related mortality (Fig. 5) was also relatively similar at 24 months between males and females (4.8% [95% CI 3.2–7.3] vs. 3.4% [95% CI 1.8–6.2], p = 0.565). Figure 6 shows the overall amputation rate over time; the log-rank test indicated a significant difference in the hazard ratio between the sexes (p = 0.009). Overall amputations were most common 6 months post-procedure, regardless of sex (38 males and 10 females). Twenty-four months post-procedure, the overall target limb amputation rate was 9.1% (95% CI 6.9–11.9) in males and 4.0% (95% CI 2.3–7.0) in females; the log-rank test revealed that the major target limb amputation rate was significantly higher (p = 0.004) in males than females (4.9% vs. 1.1%, respectively) (Supplementary Fig. 1).
Mean ABI improvements 24 months post-procedure were 0.22 ± 0.27 in males and 0.18 ± 0.26 in females (males: 0.67 ± 0.23–0.87 ± 0.22, females: 0.65 ± 0.24–0.86 ± 0.22) (Supplementary Fig. 2). Most patients improved by at least one Rutherford class in both groups (males: − 2.19 ± 1.67, females: − 2.50 ± 1.62) within 24 months (Supplementary Fig. 3). Sustained improvements in pain, reported via the pain scale and walking impairment questionnaire, were observed in both groups at 24 months (75.2% in males vs. 75.9% in females) (Supplementary Fig. 4).
Discussion
Our analysis demonstrated that 36% of participants within the BIOLUX P-III registry were female. Sex-based differences were observed, with females more commonly exhibiting CLTI (Rutherford class ≥ 4) during intervention, and males exhibiting a significantly higher rate of major target limb amputation following re-vascularisation. These findings re-emphasise the possible role of sex-based management strategies in PAD.
Several previously identified factors were proposed to explain the role of sex in PAD. First, there is a significant, sex-based difference in symptomatology; in a previous study, 9% of males and 13% of females with PAD were asymptomatic [16]. This was difficult to establish within our analysis, as asymptomatic status was an exclusion criterion. The varying presentations of atypical symptoms during assessment and subsequent delayed diagnosis and intervention in females is likely why previous studies primarily involved male participants; thus, females were recurrently under-represented. This was previously highlighted by the Walking and Leg Circulation Study cohort, which demonstrated that females were twice as likely to have atypical exertional leg symptoms when compared with males [17, 18]. Delayed diagnosis and intervention in females with PAD was also outlined within our analysis, with more females exhibiting CLTI (40.6% vs. 35.7%), and a significantly higher mean Rutherford classification (p = 0.013) during intervention.
There also appears to be several anatomical variations between sexes, with smaller vessel diameters and a more significant burden of occlusive disease in females [3]. In our analysis, occlusive disease was present in 28.4% of females and 22.9% of males. RVD was found to be significantly smaller in females (p < 0.001); correspondingly, the mean target lesion length was greater in females, although this lacked significance (p = 0.505). Despite these variations in symptomatology and underlying anatomy, the pathogenesis is believed to be comparable between sexes. The process involves vascular inflammation, decreased nitric oxide levels, increased vascular smooth muscle cell migration, and proliferation [4]; the paclitaxel coating utilised on the Passeo-18 Lux DCB aims to target some of these mechanisms to reduce the rate of neointimal hyperplasia and associated restenosis [19].
Another previously identified sex-based difference is that males with PAD have more co-morbidities than females [2]. In our analyses, coronary artery disease, diabetes mellitus, cerebrovascular disease, and dialysis dependence were more prevalent in males. Previously, this significant cardiovascular burden was identified as a major independent contributor to both overall and cardiac mortality. A recent study highlighted the propensity of males towards MACEs in such a setting [10]; interestingly, our study did not identify significant differences in overall (12.0% vs. 11.9%, p = 0.956) or cardiac-related (4.8% vs. 3.4%, p = 0.565) mortality.
Concerns regarding DCB treatment effects in females—specifically regarding TLR—arose following the PTA THUNDER trial [9], highlighting that females were twice as likely to require TLR following DCB utilisation when compared with males. In our analysis, females exhibited more advanced arterial disease at intervention (Rutherford class ≥ 4); however, clinically driven TLR was more common in males at 24 months. MAEs were also slightly more common in males, indicating that this concern may warrant further investigation and be device specific.
Males with PAD previously demonstrated a higher risk of target limb amputation [20, 21]; in our study, rates were 9.1% in males and 4.0% in females at 24 months. The reasons for this may be multifactorial. One proposed explanation is that males may not observe their feet as often as females with a subsequent delay in medical review [22]; however, this contradicts the findings within our analysis of females presenting at a later stage (CLTI: 40.6% vs. 35.7%). In our study, this increased risk of lower extremity amputation in males was further emphasised by their significantly higher rate of major target limb amputation (p = 0.004).
Rutherford classification, ABI, and pain score all significantly improved at 6, 12, and 24 months, with Rutherford classification improvements in 79.9% of males and 84.8% of females at 24 months. ABI also improved in both males (0.22 ± 0.27) and females (0.18 ± 0.26), and pain scores fell from 5.3 to 2.0 ± 2.4 in males, and 5.9 to 2.5 ± 2.6 in females during follow-up. These substantial improvements were particularly encouraging in females, as more aggressive and extensive disease status were suggested at intervention.
Limitations
The BIOLUX P-III is an all-comers registry with many limitations. First, it is prone to selection bias; since physicians enrolled patients for whom they believed therapy would be appropriate, the decision to enrol was not based on randomisation or sequential patient identification. Therefore, crural vessel disease was under-represented, reflecting the reluctance of practitioners to use DCBs for infra-popliteal lesions. Inability to cross the lesion—either primarily or via another adjunctive technique per the clinician’s discretion—resulted in exclusion from the registry, further contributing to selection bias. As this criterion was not the primary intention of the registry, this data was not captured.
Loss to follow-up was a further limitation, while the exclusion of patients with a life expectancy of < 1 year may have influenced mortality rates. No wound staging classification systems were utilised to assess the risk of amputation; such data on wound healing would have been informative for long-term prognosis. Additionally, although medication use substantially impacts outcomes, this was not formally assessed within our study; patients with PAD typically require control of optimal glucose level [23, 24], hypertension, and dyslipidaemia, with concomitant antiplatelet therapy [23].
The study was not initially designed to analyse all patient subgroups; thus, further enrolment of patients was not based on rigorous sample size calculation. Additionally, performance outcomes such as ABI, Rutherford classification assessments, or duplex ultrasound, were not available for all patients. Finally, this study did not include core laboratory evaluations, such as intraoperative angiography and/or follow-up duplex ultrasound, that may have offered meticulous quality control of our results.
Conclusion
Treatment with the Passeo-18 Lux DCB resulted in high efficacy and low complication rates. Despite there being more females with CLTI, males appeared to be associated with poorer outcomes, demonstrating a greater risk of ipsilateral major limb amputation and MAEs following DCB utilisation.
References
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–26. https://doi.org/10.1161/CIRCRESAHA.116.303849.
Hirsch A, Allison M, Gomes A, Corriere M, Duval S, Ershow A, et al. A call to action: women and peripheral artery disease: a scientific statement from the American heart association. Circulation. 2012;125:1449–72. https://doi.org/10.1016/j.jvs.2015.03.066.
Krishnan P, Tarricone A, Purushottam B, Chen S, Kapur V, Gujja K, et al. Gender differences in the outcomes of drug-coated balloon treatment in symptomatic femoropopliteal arterial disease. Vasc Endovasc Surg. 2020;54:348–54. https://doi.org/10.1177/1538574420911508.
Ramkumar N, Suckow BD, Brown JR, Sedrakyan A, Mackenzie T, Stone DH, et al. Role of sex in determining treatment type for patients undergoing endovascular lower extremity revascularization. J Am Heart Assoc. 2019;8:17. https://doi.org/10.1161/JAHA.119.013088.
Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther. 2015;22:14–21. https://doi.org/10.1177/1526602814564383.
Zeller T, Beschorner U, Pilger E, Bosiers M, Deloose K, Peeters P, et al. Paclitaxel-coated balloon in infrapopliteal arteries: 12-month results from the BIOLUX P-II randomized trial (BIOTRONIK’S-First in man study of the Passeo-18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries). JACC Cardiovasc Interv. 2015;8:1614–22. https://doi.org/10.1016/j.jcin.2015.07.011.
Tepe G, Wang J, Corpataux JM, Pua U, Binkert CA, Moscovic M, et al. BIOLUX P-III Passeo-18 Lux all-comers registry: 24-month results in below-the-knee arteries. Cardiovasc Interv Radiol. 2021;44:10–8. https://doi.org/10.1007/s00270-020-02586-3.
Schienert D, Schmidt A, Zeller T, Müller-Hülsbeck S, Sixt S, Schröder H, et al. German center subanalysis of the LEVANT 2 global randomised study of the Lutonix drug-coated balloon in the treatment of femoropopliteal occlusive disease. J Endovasc Ther. 2016;23:409–16. https://doi.org/10.1177/1526602816644592.
Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8:102–8. https://doi.org/10.1016/j.jcin.2014.07.023.
Parvar SL, Thiyagarajah A, Nerlekar N, King P, Nicholls SJ. A systematic review and meta-analysis of gender differences in long-term mortality and cardiovascular events in peripheral artery disease. J Vasc Surg. 2021;73:1456-65.e7. https://doi.org/10.1016/j.jvs.2020.09.039.
Tepe G, Zeller T, Moscovic M, Corpataux JM, Christensen JK, Keirse K, et al. Paclitaxel-coated balloon for the treatment of infrainguinal disease: 12-month outcomes in the all-comers cohort of BIOLUX P-III Global Registry. J Endovasc Ther. 2020;27:304–15. https://doi.org/10.1177/1526602819898804.
Tepe G, Zeller T, Moscovic M, Corpataux JM, Christensen JK, Keirse K, et al. Paclitaxel-coated balloon for the treatment of infra-inguinal arteries: 24-month outcomes in the full cohort of BIOLUX P-III Global Registry. Cardiovasc Interv Radiol. 2021;44:207–17. https://doi.org/10.1007/s00270-020-02663-7.
Mwipatayi BP, Barry IP, Brodmann M, Zeller T, Varcoe RL, Moscovic M, et al. Twenty-four-month outcomes of drug-coated balloon in diabetic patients in the BIOLUX P-III registry: a subgroup analysis. Ann Vasc Surg. 2021;75:237–52. https://doi.org/10.1016/j.avsg.2021.02.050.
Freisinger E, Koeppe J, Gerss J, Goerlich D, Malyar NM, Marschall U, et al. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. Eur Heart J. 2020;41:3732–9. https://doi.org/10.1093/eurheartj/ehz698.
Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7: e011245. https://doi.org/10.1161/JAHA.118.01124.
Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–91. https://doi.org/10.1016/j.jvs.2007.02.004.
McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–83. https://doi.org/10.7326/0003-4819-136-12-200206180-00008.
McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L, et al. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–8. https://doi.org/10.1046/j.1532-5415.2003.51061.x.
Cafasso D, Schneider P. How paclitaxel can improve results in diabetics. J Cardiovasc Surg (Torino). 2012;53:13–21.
Gurney JK, Stanley J, York S, Rosenbaum D, Sarfati D. Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia. 2018;61:626–35. https://doi.org/10.1007/s00125-017-4488-8.
Fan L, Wu XJ. Sex difference for the risk of amputation in diabetic patients: a systematic review and meta-analysis. PLoS ONE. 2021;16: e0243797. https://doi.org/10.1371/journal.pone.0243797.
Hämäläinen H, Rönnemaa T, Halonen JP, Toikka T. Factors predicting lower extremity amputations in patients with type 1 or type 2 diabetes mellitus: a population-based 7-year follow-up study. J Intern Med. 1999;246:97–103. https://doi.org/10.1046/j.1365-2796.1999.00523.
Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444–52. https://doi.org/10.1093/eurheartj/eht142.
Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. 2018;39:763–816. https://doi.org/10.1093/eurheartj/ehx095.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by BIOTRONIK AG, Buelach, Switzerland. The funding body was involved in the design of the study and in the collection and analysis of the data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Bibombe P Mwipatayi is a consultant with Medtronic, Getinge, Boston Scientific, and Biotronik and receives honoraria and research grants from Getinge, BD BARD, and Biotronik. Thomas Zeller (1) received honoraria from Abbott Vascular, Veryan, Biotronik, Boston Scientific Corp., Cook Medical, Gore & Associates, Medtronic, Philips-Spectranetics, Shockwave, and BIBA Medical; (2) consulted for Boston Scientific Corp., Gore & Associates, Medtronic, Veryan, Intact Vascular, Shockwave, Bayer, and Vesper Medical; (3) received research, clinical trial, or drug study funds from 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Medtronic, Philips, Terumo, TriReme, Shockwave, Med Alliance, Intact Vascular, and B. Braun; and (4) has common stock in QT Medical. Marianne Brodmann (1) received honoraria from Abbott Vascular, Biotronik, Boston Scientific Corp., Cook Medical, Medtronic, Philips-Spectranetics, Shockwave, and BD Bard and (2) consulted for Abbott Vascular, Biotronik, Boston Scientific Corp., Cook Medical, Medtronic, Philips-Spectranetics, Shockwave, BD Bard, Reflow Medical, Cagent, MedAlliance, Intact Vascular, and Bolt Medical. Gunnar Tepe received research, clinical trial, or drug study funds from Bayer Health Care, BBraun, Boston Scientific, BARD, Biotronic, Gore, Medtronic, Philips, and Shockwave. The other authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barry, I.P., Macarulay, R., Brodmann, M. et al. Sex-Related Outcomes Following Drug Balloon Angioplasty in Patients from the BIOLUX P-III Registry: A Subgroup Analysis. Cardiovasc Intervent Radiol 45, 918–928 (2022). https://doi.org/10.1007/s00270-022-03135-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03135-w