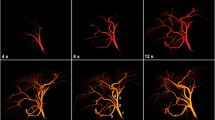
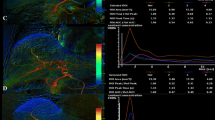
Abstract
Objective
There is no standardized and objective method for determining the optimal treatment endpoint (sub-stasis) during transarterial embolization. The objective of this study was to demonstrate the feasibility of using a quantitative digital subtraction angiography (qDSA) technique to characterize intra-procedural changes in hepatic arterial blood flow velocity in response to transarterial embolization in an in vivo porcine model.
Materials and Methods
Eight domestic swine underwent bland transarterial embolizations to partial- and sub-stasis angiographic endpoints with intraprocedural DSA acquisitions. Embolized lobes were assessed on histopathology for ischemic damage and tissue embolic particle density. Analysis of target vessels used qDSA and a commercially available color-coded DSA (ccDSA) tool to calculate blood flow velocities and time-to-peak, respectively.
Results
Blood flow velocities calculated using qDSA showed a statistically significant difference (p < 0.01) between partial- and sub-stasis endpoints, whereas time-to-peak calculated using ccDSA did not show a significant difference. During the course of embolizations, the average correlation with volume of particles delivered was larger for qDSA (− 0.86) than ccDSA (0.36). There was a statistically smaller mean squared error (p < 0.01) and larger coefficient of determination (p < 0.01) for qDSA compared to ccDSA. On pathology, the degree of embolization as calculated by qDSA had a moderate, positive correlation (p < 0.01) with the tissue embolic particle density of ischemic regions within the embolized lobe.
Conclusions
qDSA was able to quantitatively discriminate angiographic embolization endpoints and, compared to a commercially available ccDSA method, improve intra-procedural characterization of blood flow changes. Additionally, the qDSA endpoints correlated with tissue-level changes.





Similar content being viewed by others
References
Geschwind J-FH, Ramsey DE, Cleffken B, et al. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Interv Radiol. 2003;26(2):111–7.
Rhee TK, Young JY, Larson AC, et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1α in rabbit VX2 liver tumors. J Vasc Interv Radiol. 2007;18(5):639–45.
Shim J, Park J, Kim J, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99(10):2037–44.
Kobayashi N, Ishii M, Ueno Y, et al. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19(1):25–31.
Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):ajg200850181.
Jin B, Wang D, Lewandowski RJ, et al. Chemoembolization endpoints: effect on survival among patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196(4):919–28.
Lewandowski RJ, Wang D, Gehl J, et al. A comparison of chemoembolization endpoints using angiographic versus transcatheter intraarterial perfusion/MR imaging monitoring. J Vasc Interv Radiol. 2007;18(10):1249–57.
Gaba RC, Wang D, Lewandowski RJ, et al. Four-dimensional transcatheter intraarterial perfusion MR imaging for monitoring chemoembolization of hepatocellular carcinoma: preliminary results. J Vasc Interv Radiol. 2008;19(11):1589–95.
Larson AC, Wang D, Atassi B, et al. Transcatheter intraarterial perfusion: MR monitoring of chemoembolization for hepatocellular carcinoma—feasibility of initial clinical translation. Radiology. 2008;246(3):964–71.
Lin EY, Lee R-C, Guo W-Y, et al. Three-dimensional quantitative color-coding analysis of hepatic arterial flow change during chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29:1362–8.
Ionita CN, Garcia VL, Bednarek DR, et al. Effect of injection technique on temporal parametric imaging derived from digital subtraction angiography in patient specific phantoms. Proc SPIE Int Soc Opt Eng. 2014;13(9038):90380L.
Shpilfoygel SD, Close RA, Valentino DJ, et al. X-ray videodensitometric methods for blood flow and velocity measurement: a critical review of literature. Med Phys. 2000;27:2008–23.
Kennedy AS, Kleinstreuer C, Basciano CA, et al. Computer modeling of yttrium-90—microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys. 2010;76:631–7.
Wu Y, Shaughnessy G, Hoffman CA, et al. Quantification of blood velocity with 4D digital subtraction angiography using the shifted least-squares method. Am J Neuroradiol. 2018;39(10):1871–7.
Bates D, Machler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67(1). https://doi.org/10.18637/jss.v067.i01.
Dunn OJ, Clark V. Correlation coefficients measured on the same individuals. J Am Stat Assoc. 1969;64:366–77.
R Core Team. R A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019.
Chen C-W, Hsu L-S, Weng J-C, et al. Assessment of small hepatocellular carcinoma: perfusion quantification and time-concentration curve evaluation using color-coded and quantitative digital subtraction angiography. Medicine. 2018;97:e13392.
Lin Y-Y, Lee R-C, Guo W-Y, et al. Quantitative real-time fluoroscopy analysis on measurement of the hepatic arterial flow during transcatheter arterial chemoembolization of hepatocellular carcinoma: comparison with quantitative digital subtraction angiography analysis. Cardio Vasc Interv Radiol. 2016;39:1557–63.
Meram E, Harari C, Shaughnessy G, et al. Quantitative 4D-digital subtraction angiography to assess changes in hepatic arterial flow during transarterial embolization: a feasibility study in a swine model. J Vasc Interv Radiol. 2019;30(8):1286–92.
Meram E, Shaughnessy G, Longhurst C, et al. Optimization of quantitative 4D-digital subtraction angiography in a porcine liver model. Eur Radiol Exp. 2020;4:1–10.
Ghibes P, Hefferman G, Nikolaou K, et al. Quantitative evaluation of peripheral arterial blood flow using peri-interventional fluoroscopic parameters: an in vivo study evaluating feasibility and clinical utility. Biomed Res Int. 2020;2020:9526790.
Acknowledgements
Sarvesh Periyasamy is supported by an MD-Ph.D Graduate Fellowship through the University of Wisconsin School of Medicine and Public Health, Department of Radiology, an NCI Ruth L. Kirschstein NRSA Fellowship 1F30CA250408-01, and an MSTP NIH Grant T32GM008692. Carson Hoffman is supported by the Radiological Sciences Training Grant (NIH Grant T32CA009206 through the National Cancer Institute). Portions of the image processing were performed on a GPU donated by the NVIDIA Corporation.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Michael A. Speidel received a grant from Siemens Healthineers. Author Paul F. Laeseke is a consultant for Neuwave Medical/Ethicon, a consultant and shareholder for Elucent Medical, a shareholder for Histosonics, and a shareholder for McGinley Orthopeadic Innovations. For the remaining authors, no other conflicts were declared.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Periyasamy, S., Hoffman, C.A., Longhurst, C. et al. A Quantitative Digital Subtraction Angiography Technique for Characterizing Reduction in Hepatic Arterial Blood Flow During Transarterial Embolization. Cardiovasc Intervent Radiol 44, 310–317 (2021). https://doi.org/10.1007/s00270-020-02640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02640-0