Abstract
Purpose
To evalutate safety and efficacy of degradable starch microspheres (DSM) as embolic agent in transarterial chemoembolisation (TACE) of unresectable, locally extensive hepatocellular carcinoma (HCC).
Materials and Methods
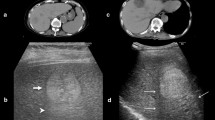
In this retrospective study, 37 patients with intermediate to advanced HCC treated with ≥ 3 chemoembolisations with doxorubicin/epirubicin and DSM were analysed. Patients were treated with three consecutive chemoembolisations in 4-weekly intervals. Clinical parameters and laboratory findings were obtained from patient records before and after each intervention. Tumour response was assessed after every 3 embolisations by CT/MRI according to modified response evaluation criteria in solid tumours.
Results
Thirty-seven patients with HCC were treated with 177 DSM-TACEs (3–12/patient, mean 4.8). Disease stages according to the Barcelona Clinic Liver Cancer (BCLC) staging system were: 27 × B, 9 × C, 1 × D. Five patients had uninodular, 32 multinodular (23 bilobar) disease. Three patients had portal vein invasion. Apart from one possibly procedure-related grade 3 complication, only grade 1 adverse events occurred. These were pain reacting to analgesics (23%), transient nausea (11%), vomiting (3%) and post-embolisation syndrome (4%). Transient laboratory changes were bone marrow toxicity (29%) and increase in INR (14%), creatinine (8%) or bilirubin (38%). Tumour response was objective response rate 49%, disease control rate 83%. Median survival was 19 months: 22 months for BCLC stage B and 6.7 months for BCLC stages C + D. Responders had a significantly better prognosis than non-responders.
Conclusion
DSM-TACE of HCC is safe even in patients with advanced disease stages. Tumour response and survival rates were encouraging in our series of patients with locally extensive disease.


Similar content being viewed by others
References
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Lo C-M, Ngan H, Tso W-K, Liu C-L, Lam C-M, Poon RT-P, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S179–88.
Aronsen KF, Hellekant C, Holmberg J, Rothman U, Teder H. Controlled blocking of hepatic artery flow with enzymatically degradable microspheres combined with oncolytic drugs. Eur Surg Res. 1979;11(2):99–106.
Forsberg JO. Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres. Experiments on rats. Acta Chir Scand. 1978;144(5):275–81.
Lindell B, Aronsen KF, Rothman U. Repeated arterial embolization of rat livers by degradable microspheres. Eur Surg Res. 1977;9(5):347–56.
Lote K, Myking AO. Starch microsphere induced small intestinal ischaemia. Blood flow and morphologic investigations of late effects. Acta Radiol Oncol. 1982;21(5):353–8.
Dakhil S, Ensminger W, Cho K, Niederhuber J, Doan K, Wheeler R. Improved regional selectivity of hepatic arterial BCNU with degradable microspheres. Cancer. 1982;50(4):631–5.
Ebert M, Ebert J, Berger G. Intravital microscopic research of microembolization with degradable starch microspheres. J Drug Deliv. 2013;2013:242060.
Håkansson L, Håkansson A, Morales O, Thorelius L, Warfving T. Spherex (degradable starch microspheres) chemo-occlusion–enhancement of tumor drug concentration and therapeutic efficacy: an overview. Semin Oncol. 1997;24(2 Suppl 6):S6-100–9.
Sigurdson ER, Ridge JA, Daly JM. Intra-arterial infusion of doxorubicin with degradable starch microspheres. Improvement of hepatic tumor drug uptake. Arch Surg. 1986;121(11):1277–81.
Li X, Feng G-S, Zheng C-S, Zhuo C-K, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–82.
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–9.
Xiao E-H, Guo D, Bian D-J. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15(36):4582–6.
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind J-FH. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29(30):3960–7.
Ye W. The complexity of translating anti-angiogenesis therapy from basic science to the clinic. Dev Cell. 2016;37(2):114–25.
Niessen C, Unterpaintner E, Goessmann H, Schlitt HJ, Mueller-Schilling M, Wohlgemuth WA, et al. Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25(2):240–7.
Iezzi R, Pompili M, Nestola M, Siciliano M, Annicchiarico E, Zocco MA, et al. Transarterial chemoembolization with degradable starch microspheres (DSM-TACE): an alternative option for advanced HCC patients? Preliminary results. Eur Rev Med Pharmacol Sci. 2016;20(13):2872–7.
Yamasaki T, Hamabe S, Saeki I, Harima Y, Yamaguchi Y, Uchida K, et al. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol. 2011;46(3):359–66.
de Baere T, Arai Y, Lencioni R, Geschwind J-F, Rilling W, Salem R, et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Interv Radiol. 2016;39(3):334–43.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. US Department of Health and Human Services; National Institutes of Health, National Cancer Institute. 2010
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Interv Radiol. 2017;40(8):1141–6.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35(2):371–86.
Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Interv Radiol. 2007;30(1):6–25.
Craig P, Young S, Golzarian J. Current trends in the treatment of hepatocellular carcinoma with transarterial embolization: variability in technical aspects. Cardiovasc Interv Radiol. 2019;42(9):1322–8.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol. 2010;33(1):41–52.
Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24(2):161–9.
Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–64.
Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24(4):490–500.
Kudo M, Han G, Finn RS, Poon RTP, Blanc J-F, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–707.
Gruber-Rouh T, Schmitt C, Naguib NNN, Nour-Eldin NA, Eichler K, Beeres M, et al. Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: comparative study. BMC Cancer. 2018;18(1):188.
Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34(17):2046–53.
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind J-FH. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–16.
Orlacchio A, Chegai F, Francioso S, Merolla S, Monti S, Angelico M, et al. Repeated transarterial chemoembolization with degradable starch microspheres (DSMs-TACE) of unresectable hepatocellular carcinoma: a prospective pilot study. Curr Med Imaging Rev. 2018;14(4):637–45.
Schicho A, Pereira PL, Haimerl M, Niessen C, Michalik K, Beyer LP, et al. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget. 2017;8(42):72613–20.
Kirchhoff TD, Rudolph KL, Layer G, Chavan A, Greten TF, Rosenthal H, et al. Chemoocclusion vs chemoperfusion for treatment of advanced hepatocellular carcinoma: a randomised trial. Eur J Surg Oncol. 2006;32(2):201–7.
Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(6):1330–5.
Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Interv Radiol. 2012;35(5):1119–28.
Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, et al. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26(2):159–64.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alexander Gross has received travel grants and a speaker honorarium from PharmaCept GmbH (Berlin, Germany). Thomas Albrecht has received travel grants and speaker honoraria from PharmaCept GmbH (Berlin, Germany).
Consent for Publication
The study was approved by the local ethics committee (Ethikkommission der Ärztekammer Berlin).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. The study was approved by the local ethics committee (Ethikkommission der Ärztekammer Berlin).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gross, A., Albrecht, T. Transarterial Chemoembolisation (TACE) with Degradable Starch Microspheres (DSM) and Anthracycline in Patients with Locally Extensive Hepatocellular Carcinoma (HCC): Safety and Efficacy. Cardiovasc Intervent Radiol 43, 402–410 (2020). https://doi.org/10.1007/s00270-019-02364-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02364-w