Abstract
Background
We designed a novel transcatheter arterial infusion chemotherapy (TAI) using iodized oil (lipiodol) and degradable starch microspheres (DSM) for hepatocellular carcinoma (HCC) patients. In this study, we investigated the efficacy of TAI using lipiodol and DSM in a prospective randomized trial.
Methods
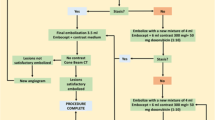
We randomly divided 45 patients with HCC into 3 groups: TAI using lipiodol (lipiodol group, n = 15), TAI using DSM (DSM group, n = 15), and TAI using lipiodol and DSM (lipiodol + DSM group, n = 15). In the lipiodol group, a mixture of cisplatin and lipiodol was administered. In the DSM group, a mixture of cisplatin and DSM was administered. In the lipiodol + DSM group, a mixture of cisplatin and lipiodol was administered, followed by DSM.
Results
The response rates were 40% in the lipiodol group, 53.4% in the DSM group, and 80% in the lipiodol + DSM group, respectively. The response rate tended to improve in the lipiodol + DSM group (lipiodol group vs. lipiodol + DSM group, P = 0.07). The median progression-free survival time was 177 days in the lipiodol group, 287 days in the DSM group, and 377 days in the lipiodol + DSM group. The progression-free survival in the lipiodol + DSM group was significantly better than those in the DSM group (P = 0.020) and the lipiodol group (P = 0.035). There were no serious adverse effects among the 3 groups.
Conclusions
TAI using lipiodol and DSM was superior to TAI using lipiodol only and TAI using DSM only because of improvements in therapeutic effects and progression-free survival.



Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–26.
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34.
Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe. 1980–2004. Hepatology. 2008;48:137–45.
El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–63.
Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–64.
Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83.
Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342–8.
Todo S, Furukawa H, Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–61.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25.
Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–83.
Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Thong D, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. J Hepatol. 1998;29:129–34.
Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Moriyama N, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681–8.
Yoon HJ, Kim JH, Kim KA, Lee IS, Ko GY, Song HY, et al. Transcatheter arterial chemo-lipiodol infusion for unresectable hepatocellular carcinoma in 96 high-risk patients. Clin Radiol. 2010;65:271–7.
Ikeda M, Maeda S, Shibata J, Muta R, Ashihara H, Tanaka M, et al. Transcatheter arterial chemotherapy with and without embolization in patients with hepatocellular carcinoma. Oncology. 2004;66:24–31.
Forsberg JO. Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres. Experiments on rats. Acta Chir Scand. 1978;144:275–81.
Lindell B, Aronsen KF, Rothman U. Repeated arterial embolization of rat livers by degradable microspheres. Eur Surg Res. 1977;9:347–56.
Dakhil S, Ensminger W, Cho K, Niederhuber J, Doan K, Wheeler R. Improved regional selectivity of hepatic arterial BCNU with degradable microspheres. Cancer. 1982;50:631–5.
Ensminger WD, Gyves JW, Stetson P, Walker-Andrews S. Phase I study of hepatic arterial degradable starch microspheres and mitomycin. Cancer Res. 1985;45:4464–7.
Taguchi T. Chemo-occlusion for the treatment of liver cancer. A new technique using degradable starch microspheres. Clin Pharmacokinet. 1994;26:275–91.
Carr BI, Zajiko A, Bron K, Orons P, Sammon J, Baron R. Phase II study of Spherex (degradable starch microspheres) injected into the hepatic artery in conjunction with doxorubicin and cisplatin in the treatment of advanced-stage hepatocellular carcinoma: interim analysis. Semin Oncol. 1997;24(suppl 6):S6-97–9.
Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, et al. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26:159–64.
Kirchhoff TD, Rudolph KL, Layer G, Chavan A, Greten TF, Rosenthal H, et al. Chemoocclusion vs. chemoperfusion for treatment of advanced hepatocellular carcinoma: a randomised trial. Eur J Surg Oncol. 2006;32:201–7.
Yamasaki T, Saeki I, Harima Y, Okita K, Segawa M, Yamaguchi Y, et al. The pilot study: a novel transarterial chemoembolization using degradable starch microspheres for hepatocellular carcinoma (in Japanese with English abstract). Kanzo. 2008;49:25–7.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 5th edn. Tokyo: Kanehara, 2009 (in Japanese).
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207–15.
Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Yokoyama R, et al. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. AJR Am J Roentgenol. 1997;168:1193–8.
Okusaka T, Okada S, Ishii H, Ikeda M, Nakasuka H, Nagahama H, et al. Transarterial chemotherapy with zinostatin stimalamer for hepatocellular carcinoma. Oncology. 1998;55:276–83.
Takayasu K, Arri S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704.
Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293–9.
National Cancer Institute (2009) Common Terminology Criteria for Adverse Events v.4.0. National Cancer Institute, Bethesda
Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–81.
Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, et al. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564–8.
Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38:474–83.
Vogl TJ, Trapp M, Shroeder H, Mack M, Schuster A, Schmitt J, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success—results from a liver transplantation center. Radiology. 2000;214:349–57.
Kirchhoff TD, Bleck JS, Dettmer A, Chavan A, Rosenthal H, Merkesdal S, et al. Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int. 2007;6:259–66.
Kanematsu M. Transcatheter arterial chemoembolization therapy with epirubicin hydrochloride, mitomycin C-iohexol-lipiodol emulsion (EMILE) for hepatocellular carcinoma. J Gastroenterol. 1995;30:215–23.
Sigurdson ER, Ridge JA, Daly JM. Intra-arterial infusion of doxorubicin with degradable starch microspheres. Improvement of hepatic tumor drug uptake. Arch Surg. 1986;121:1277–81.
Nakamura H, Hashimoto T, Oi H, Sawada S. Iodized oil in the portal vein after arterial embolization. Radiology. 1988;167:415–7.
Kan Z, Ivancev K, Hägerstrand I, Chuang VP, Lunderquist A. In vivo microscopy of the liver after injection of lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419–25.
Acknowledgment
This study was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (Grant No. 20591478).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamasaki, T., Hamabe, S., Saeki, I. et al. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 46, 359–366 (2011). https://doi.org/10.1007/s00535-010-0306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0306-5