Abstract
Background
This study aimed to evaluate the embolic properties, time to reperfusion, and histologic changes in temporary embolization of liver tissue with degradable starch microspheres (DSM) in a swine model.
Methods
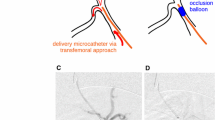
In four adult minipigs, DSMs were injected into the right or left hepatic artery on the lobar level until complete stasis of the blood flow was detectable angiographically. The time required to complete angiographically determined reperfusion was noted. The animals were killed 3 h after complete reperfusion, and samples were taken from the liver. Histologic examinations of the embolized liver parenchyma and untreated tissue were performed.
Results
Hepatic arterial embolization using DSMs was technically successful in all cases, with complete blood flow stasis shown by control angiography. A single vial of DSMs (450 mg/7.5 ml) was sufficient to embolize a whole liver lobe in all cases. Angiography showed complete reconstitution of hepatic arterial perfusion after a mean time to reperfusion of 32 ± 6.1 min (range, 26–39 min). Hematoxylin and eosin staining showed no histologically detectable differences between untreated tissue and parenchyma embolized with DSMs except for mild sinusoidal congestion in one case. Indirect in situ DNA nick end labeling staining (TUNEL) showed only single positive hepatocytes, indicating apoptosis.
Conclusion
Temporary embolization of the hepatic artery using DSMs is feasible with complete reperfusion after 30 min in pigs. Even after complete arterial blood flow stasis, no extensive tissue damage to the embolized liver parenchyma was observed at histologic examinations in this short-term study.




Similar content being viewed by others
References
Murata S, Mine T, Ueda T, Nakazawa K, Onozawa S, Yasui D, Kumita S (2013) Transcatheter arterial chemoembolization based on hepatic hemodynamics for hepatocellular carcinoma. Sci World J 2013:479805
de Baere T, Deschamps F (2011) Arterial therapies of colorectal cancer metastases to the liver. Abdom Imaging 36(6):661–670
Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind JF et al (2005) Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol 16:205–213
Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O et al (2005) Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 16:1627–1639
Lencioni R, Petruzzi P, Crocetti L (2013) Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol 30:3–11
Sueyoshi E, Hayashida T, Sakamoto I, Uetani M (2010) Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol 195(1):245–252
Wiggermann P, Wohlgemuth WA, Heibl M, Vasilj A, Loss M, Schreyer AG, Stroszczynski C, Jung EM (2013) Dynamic evaluation and quantification of microvascularization during degradable starch microspheres transarterial chemoembolisation (DSM-TACE) of HCC lesions using contrast enhanced ultrasound (CEUS): a feasibility study. Clin Hemorheol Microcirc 53(4):337–348
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146(6):873–887
Nishiofuku H, Tanaka T, Matsuoka M, Otsuji T, Anai H, Sueyoshi S, Inaba Y, Koyama F, Sho M, Nakajima Y, Kichikawa K (2013) Transcatheter arterial chemoembolization using cisplatin powder mixed with degradable starch microspheres for colorectal liver metastases after FOLFOX failure: results of a phase I/II study. J Vasc Interv Radiol 24(1):56–65
Murata S, Tajima H, Ichikawa K, Onozawa S, Wang J, Kumita S, Nomura K (2008) Oily chemoembolization combined with degradable starch microspheres for HCC with cirrhosis. Hepatogastroenterology 55(84):1041–1046
Meyer C, Pieper CC, Ezziddin S, Wilhelm KE, Schild HH, Ahmadzadehfar H (2014) Feasibility of temporary protective embolization of normal liver tissue using degradable starch microspheres during radioembolization of liver tumours. Eur J Nucl Med Mol Imaging 41(2):231–237
Wang J, Murata S, Kumazaki T (2006) Liver microcirculation after hepatic artery embolization with degradable starch microspheres in vivo. World J Gastroenterol 12:4214–4218
Forsberg JO (1978) Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres: experiments on rats. Acta Chir Scand 144:275–281
Yamasaki T, Hamabe S, Saeki I, Harima Y, Yamaguchi Y, Uchida K, Terai S, Sakaida I (2011) A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 46(3):359–366
Pohlen U, Buhr HJ, Berger G, Ritz JP, Holmer C (2012) Hepatic arterial infusion (HAI) with PEGylated liposomes containing 5-FU improves tumor control of liver metastases in a rat model. Invest New Drugs 30:927–935
Chang D, Jenkins SA, Grime SJ, Nott DM, Cooke T (1996) Increasing hepatic arterial flow to hypovascular hepatic tumours using degradable starch microspheres. Br J Cancer 73(8):961–965
Ritz JP, Lehmann K, Isbert C, Roggan A, Germer CT, Buhr HJ (2005) Effectivity of laser-induced thermotherapy: in vivo comparison of arterial microembolization and complete hepatic inflow occlusion. Lasers Surg Med 36(3):238–244
Ritz JP, Lehmann KS, Zurbuchen U, Wacker F, Brehm F, Isbert C, Germer CT, Buhr HJ, Holmer C (2007) Improving laser-induced thermotherapy of liver metastases: effects of arterial microembolization and complete blood flow occlusion. Eur J Surg Oncol 33(5):608–615
Monbaliu D, Crabbé T, Roskams T, Fevery J, Verwaest C, Pirenne J (2005) Livers from non–heart-beating donors tolerate short periods of warm ischemia. Transplantation 79(9):1226–1230
Wiggermann P, Heibl M, Niessen C, Muller-Wille R, Gossmann H, Uller W et al (2012) Degradable starch microspheres transarterial chemoembolisation (DSM-TACE) of HCC: dynamic contrast-enhanced ultrasonography (DCE-US) based evaluation of therapeutic efficacy using a novel perfusion software. Clin Hemorheol Microcirc 52:123–129
Tanabe T, Fujii S (2000) Investigation of basal levels of blood biochemistry in swine. Annual Report of Kyoto City Institute of Health and Environmental Sciences, vol 66. Kyōto-shi: Dō Kenkyūjo, Kyoto, Japan, pp 89–96
Latimer KS, Mahaffey EA, Prasse KW (2003) Duncan and Prasse’s veterinary laboratory medicine: clinical pathology, 4th edn. Wiley-Blackwell, Ames
Yipintsoi T, Dobbs WA Jr, Scanlon PD, Knopp TJ, Bassingthwaighte JB (1973) Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res 33(5):573–587
Domenech RJ, Hoffman JI, Noble MI, Saunders KB, Henson JR, Subijanto S (1969) Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res 25(5):581–596
Bastian P, Bartkowski R, Köhler H, Kissel T (1998) Chemo-embolization of experimental liver metastases: part I. distribution of biodegradable microspheres of different sizes in an animal model for the locoregional therapy. Eur J Pharm Biopharm 46(3):243–254
Yamada T, Nakamura K, Matsuo R, Murata K, Usuki N, Kaminou T, Tsubakimoto M, Manabe T, Takashima S, Nakatsuka H et al (1988) Experimental and clinical studies on degradable starch microspheres (DSM) in the treatment of hepatic neoplasms. Gan To Kagaku Ryoho 15(8 Pt 2):2617–2620
Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, Sakai H, Mera K, Maru Y, Yoshino M (2003) Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol 26(2):159–164
Kan Z, Madoff DC (2008) Liver anatomy: microcirculation of the liver. Semin Intervent Radiol 25(2):77–85
Conflict of interest
Claus C. Pieper, Carsten Meyer, Brigitte Vollmar, Karlheinz Hauenstein, Hans H. Schild, and Kai E. Wilhelm have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pieper, C.C., Meyer, C., Vollmar, B. et al. Temporary Arterial Embolization of Liver Parenchyma with Degradable Starch Microspheres (EmboCept®S) in a Swine Model. Cardiovasc Intervent Radiol 38, 435–441 (2015). https://doi.org/10.1007/s00270-014-0966-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0966-2