Abstract
Transcatheter arterial chemoembolization (TACE) offers a survival benefit to patients with intermediate hepatocellular carcinoma (HCC). A widely accepted TACE regimen includes administration of doxorubicin-oil emulsion followed by gelatine sponge—conventional TACE. Recently, a drug-eluting bead (DC Bead®) has been developed to enhance tumor drug delivery and reduce systemic availability. This randomized trial compares conventional TACE (cTACE) with TACE with DC Bead for the treatment of cirrhotic patients with HCC. Two hundred twelve patients with Child-Pugh A/B cirrhosis and large and/or multinodular, unresectable, N0, M0 HCCs were randomized to receive TACE with DC Bead loaded with doxorubicin or cTACE with doxorubicin. Randomization was stratified according to Child-Pugh status (A/B), performance status (ECOG 0/1), bilobar disease (yes/no), and prior curative treatment (yes/no). The primary endpoint was tumor response (EASL) at 6 months following independent, blinded review of MRI studies. The drug-eluting bead group showed higher rates of complete response, objective response, and disease control compared with the cTACE group (27% vs. 22%, 52% vs. 44%, and 63% vs. 52%, respectively). The hypothesis of superiority was not met (one-sided P = 0.11). However, patients with Child-Pugh B, ECOG 1, bilobar disease, and recurrent disease showed a significant increase in objective response (P = 0.038) compared to cTACE. DC Bead was associated with improved tolerability, with a significant reduction in serious liver toxicity (P < 0.001) and a significantly lower rate of doxorubicin-related side effects (P = 0.0001). TACE with DC Bead and doxorubicin is safe and effective in the treatment of HCC and offers a benefit to patients with more advanced disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is an increasingly common tumor with a poor prognosis and limited systemic treatment options; approximately 80% of patients die within a year of diagnosis. In men, it is the fifth most common cancer worldwide and the third leading cause of cancer-related death [1, 2].
Barcelona Clinic Liver Cancer (BCLC) tumor staging [3, 4] combines the stage of liver disease, tumor stage, clinical performance, and treatment options and is endorsed by the European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Disease (AASLD) [5]. In countries where no systematic screening of cirrhotic patients is performed, 50–75% are diagnosed when HCC is at an advanced stage (BCLC Stage C, Child-Pugh A/B, cancer symptoms present, and/or vascular invasion and extrahepatic spread) [6]. Such patients are precluded from surgery, and for many years doxorubicin had been used for systemic treatment [5], albeit without a proven survival benefit [7]. Thus, there is interest in new antiangiogenic agents. Recent data have established Sorafenib as the preferred systemic therapy for advanced HCC [8]. For unresectable intermediate-stage HCC (BCLC Stage B, Child-Pugh A/B, with large or multifocal HCC, no vascular invasion or extrahepatic spread), the current standard treatment is transarterial chemoembolization (TACE) [6].
TACE involves the periodic injection of a chemotherapeutic agent, mixed with embolic material, administered selectively into the feeding arteries of the tumor to potentially obtain higher intratumor drug concentrations compared to intravenous therapy, with occlusion of the blood vessel causing infarction and necrosis [9]. In HCC patients, TACE has achieved partial responses in up to 62% of patients, as well as significantly delayed tumor progression and vascular invasion [10–16]. Llovet et al. were the first to show a statistically significant benefit in survival for chemoembolization using doxorubicin (50–75 mg/m2) and Gelfoam compared with best supportive care (BSC) [16]. Although a survival benefit of TACE over symptomatic treatment or systematic chemotherapy was demonstrated in a meta-analysis of randomized controlled trials, overall survival at 3 years remained low (<30%) for intermediate HCC patients [17]. A further review failed to demonstrate either a survival difference between TACE and embolization alone or superiority of one chemotherapeutic agent over another [18]. Post-TACE complications, e.g., acute liver or renal failure, encephalopathy, ascites, and upper gastrointestinal bleeding, may be severe [18]. There is therefore a requirement for treatment regimens that improve response rates and survival, while reducing the risk of post-TACE complications.
The DC Bead (Biocompatibles UK Ltd.) is a novel drug delivery embolization system, comprising biocompatible, nonresorbable hydrogel beads capable of being loaded with anthracyclin derivatives such as doxorubicin [19, 20], the most widely used chemotherapeutic for the treatment of HCC. Preclinical [21, 22] and clinical [23, 24] studies have shown that TACE with DC Bead results in higher tumor concentrations and lower systemic concentrations of doxorubicin compared to intra-arterial doxorubicin and conventional TACE. Phase I/II studies in HCC have demonstrated promising efficacy with low toxicity [23–26].
This phase II study is the first comparative study designed to evaluate the safety and efficacy of DC Bead with doxorubicin in the treatment of HCC in comparison with conventional TACE (cTACE), the current standard treatment [18].
Materials and Methods
Patients
Patients aged >18 years with HCC unsuitable for resection or percutaneous ablation, (BCLC A/B, without portal invasion or extrahepatic spread) were eligible for the study. Eligibility criteria also included: no previous chemotherapy, radiotherapy or transarterial embolization (with or without chemotherapy), a confirmed diagnosis of HCC according to EASL, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and preserved liver function (Child-Pugh Class A or B). Patients were excluded if they had another primary tumor, advanced liver disease (bilirubin levels >3 mg/dl, AST or ALT >5 × upper limit of normal or >250 U/l), advanced tumoral disease (vascular invasion or extrahepatic spread, or diffuse HCC, defined as >50% liver involvement), or contraindications for doxorubicin administration.
All patients provided written informed consent. The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guideline on Good Clinical Practice, and relevant local laws and regulations. Ethics committee approval was obtained. Independent Data and Safety Monitoring Committees were established to monitor efficacy and safety data. The study was registered at www.clinicaltrials.gov (NCT00261378), conducted according to the HCC Clinical Trial Design guidelines [27], and reported according to CONSORT recommendations [28, 29].
Study Design
This was an international, multicenter, prospective, randomized, single-blind, phase II study. Patients were randomized (1:1) to receive doxorubicin via TACE with DC Bead or conventional TACE (cTACE). Randomization was centralized, with stratification factors of Child-Pugh class (A/B), ECOG performance status (0/1), prior curative (resection or percutaneous ablation) treatment (yes/no), and bilobar disease (yes/no), representing more advanced disease. Randomized treatment allocation was predetermined by an independent statistician and used a randomized permuted block design to ensure that, at the conclusion of the study treatment, group sizes were similar both overall and for each level of stratification factor. The randomization was integrated into the web-based Case Report Form after screening.
Dose selection was based on cTACE treatment protocols of doxorubicin, 50–75 mg/m2. Patients typically receive between 100 and 150 mg in a single treatment session. In two previous dose escalation studies [23, 24], DC Bead loaded with doxorubicin in the range from 25 to 150 mg was shown to be safe and effective even at the highest dose of 150 mg.
In this study, patients in the DC Bead group received 4 ml DC Bead (1 vial of 300–500 μm first, followed by 1 vial of 500–700 μm) loaded with doxorubicin (150 mg per procedure) mixed with nonionic contrast medium. Lipiodol (iodinated poppy seed oil; Guerbet, France) was not used. No dose adjustment was made for bilirubin concentration or body surface area. In the cTACE group, patients received an intra-arterial injection of an emulsion of doxorubicin (50–75 m2 to a maximum of 150 mg, adjusted for bilirubin concentration and body surface area) in lipiodol followed by particle embolization with an embolic agent of the investigator’s choice (Gelfoam particles, Embosphere, Contour SE, Bead Block, PVA particles). In both treatment groups, patients were treated at 2-monhtly intervals [25, 26], received a maximum of three chemoembolizations (at baseline, 2 months, and 4 months), and were followed for 6 months. For patients with bilobar disease who could not be treated superselectively in a single treatment, a second embolization, of the alternative lobe, was performed within 3 weeks of the first procedure (procedure 1B) provided that there was no contraindication due to systemic toxicity and/or clinical performance. Reasons for discontinuation of treatment included ineligibility prior to chemoembolization, progressive disease, and severe systemic toxicity.
In both treatment arms, catheterization was performed via a femoral artery, and superselective embolization of the hepatic artery branches feeding the tumor was performed. The embolization endpoint was defined as stasis in the second- or third-order branches of the right or left hepatic artery. A microcatheter could be used to select a branch feeding the tumor.
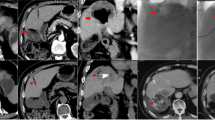
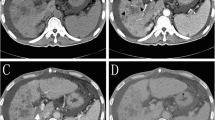
Tumor response according to EASL criteria was evaluated by magnetic resonance imaging (MRI) performed at baseline and 1, 3, and 6 months (Fig. 1). MRI was performed on 1.5-T scanners using a spoiled gradient-echo T1-weighted sequence and fast spin-echo T2-weighted sequence with fat suppression. A dynamic multiphasic, contrast-enhanced, spoiled gradient-echo T1-weighted sequence with arterial, portal, equilibrium, and delayed phase was performed. MRI scans were assessed independently by two assessors blinded to treatment allocation (followed by adjudication in case of disagreement).
Study Hypothesis and Objective
The hypothesis was that treatment of HCC with DC Bead is superior to treatment with cTACE. The objective was to evaluate the safety and 6-month tumor response of chemoembolization with DC Bead vs. cTACE.
Outcomes and Procedures/Assessments
Efficacy
The primary efficacy endpoint was the 6-month tumor response rate, according to the amended EASL response criteria, an accepted method for assessing tumor necrosis following locoregional therapy [30]. According to this amendment, response of target lesions is classified according to the presence and the dimensions of the viable tumor, defined as a tumor that takes up contrast in the arterial phase of radiologic imaging. The primary readers manually traced viable tumor on MRI slices obtained at baseline and after treatment, and computer calculations were made to quantify percentage changes in viable tumor volume and therefore to define the response of target lesions. The emergence of one or more new lesions was considered evidence of progression in the overall patient response assessment, regardless of the response obtained in target lesions. Objective response rate (OR) was defined as complete response plus partial response, and disease control rate as OR plus stable disease.
Safety
The primary safety endpoint was the incidence of treatment-related serious adverse events (SAEs) occurring within 30 days of a treatment procedure. Secondary safety outcomes included the incidence and severity of adverse events (AEs) and SAEs, liver function parameters, laboratory abnormalities, and cardiac function (ejection fraction).
Statistical Analysis
The study aimed to reject with 80% power, at a one-sided significance level of α = 0.025, the null hypothesis H0, i.e., πDC Bead = πcTACE against the alternative hypothesis, H1, i.e., πDC Bead > πcTACE, where π denotes the rate of patients in the respective treatment arm with an objective tumor response at 6 months. The study was conducted using an adaptive three-stage group sequential test design within the Δ class of critical values from Wang and Tsiatis [31]. Assuming objective tumor response rates of 55% (DC Bead) and 35% (cTACE), and a total n = 200, the statistical power was approximately 81.3%. Interim analyses were conducted independently and results kept confidential until completion of the trial. Following each interim analysis, the Data Monitoring Committee made recommendations to continue, modify, or stop the trial, based on interim analysis results (stopping criteria at n1 = 60, boundary P-value = 0.00015; n2 = 40, boundary P-value = 0.00258; n3 = 100, boundary P-value = 0.02396).
The primary analysis of efficacy was based on the Modified Intention-to-Treat (MITT) population, defined as all randomized patients who received at least one chemoembolization; this also defined the safety population. The primary safety endpoint was analyzed using the chi-square test and all safety data are presented descriptively.
All other group comparisons were supportive in nature and the secondary efficacy endpoints were analyzed descriptively using appropriate statistical methods for each endpoint. Where statistical modeling was used, baseline stratification factors were included. All endpoints were also presented using descriptive statistical methods and all statistical testing was two-sided at the 5% level of significance. The preplanned analyses of the subgroup of more advanced patients (Child-Pugh B, ECOG 1, bilobar disease, recurrent disease) revealed a clinically relevant trend toward a higher OR rate for DC Bead over cTACE. Supplementary post hoc analyses focused on treatment response and safety were therefore undertaken. The frequency of doxorubicin-related AEs, and of overall AEs, and the level of liver toxicity were explored further. These data were hypothesis-generating in nature, and P-values should be interpreted in the exploratory sense.
Results
Patients were enrolled at 19 centers in five countries (France, Germany, Switzerland, Austria, and Greece) between 25 November 2005 and 27 June 2007. A total of 212 patients were randomized to TACE with DC Bead (n = 102) or cTACE (n = 110). Due to dropouts prior to first treatment, the MITT population included 93 and 108 patients. The last-observation-carried-forward (LOCF) principle was used so that the primary endpoint could be assessed using the full MITT population (Fig. 1). At baseline there were no major differences between the treatment groups with regard to patient demographics, tumor burden, or health status (Table 1). The majority of patients (66.7%) in both groups were considered more advanced, as they met the higher risk criteria for one or more of the four prognostic factors, i.e., Child Pugh B, ECOG 1, bilobar or recurrent disease (63 of 93 DC Bead and 72 of 108 cTACE patients). The mean total dose of doxorubicin administered was higher in the DC Bead group compared with the cTACE group (295 vs. 223 mg) and in all subgroups. The mean volume of lipiodol administered was 10 ml per treatment in the cTACE group [32]. The numbers of chemoembolizations in each treatment group were similar: 93 and 108 patients, respectively, received a first; 82% of patients in each group received a second; and 61% and 57%, respectively, received a third chemoembolization.
Efficacy (Tumor Response—EASL)
At 6 months, a complete response was achieved in 25 (26.9%) vs. 24 (22.2%) patients, a partial response in 23 (24.7%) vs. 23 (21.3%) patients, and stable disease in 11 (11.8%) vs. 9 (8.3%) patients in the DC Bead vs. cTACE arm, respectively. Progressive disease was observed in 30 (32.3%) vs. 44 (40.7%) patients, respectively; 4 DC Bead patients and 8 cTACE patients withdrew prior to the first MRI scan. Reasons for these withdrawals were AEs (four DC Bead and four cTACE), withdrawn consent (two cTACE), and post consent ineligibility (two cTACE). Therefore, the OR rate was 51.6% vs. 43.5% in the DC Bead vs. cTACE arm, respectively; the hypothesis of superiority was not met (one-sided P = 0.11) (Fig. 2). The difference between groups in favor of DC Bead was 8.1% (two-sided 95% repeated confidence interval (RCI), −4.8 to 22.6%). The disease control rates were 63.4% vs. 51.9%, respectively (two-sided P = 0.11). Boundary P-values were not exceeded in the interim analyses.
Tumor response at 6 months (LOCF) (MITT population and advanced patient group*, **). * More advanced disease was at least one of Child-Pugh B, ECOG 1, undergone prior curative treatment (i.e., recurrent disease), and presence of bilobar disease. In accordance with the EASL criteria: complete response (CR)—complete disappearance of all known viable tumor (assessed via uptake of contrast in the arterial phase of the MRI scan) and no new lesions; partial response (PR)—50% reduction in viable tumor area of all measurable lesions; stable disease (SD)—all other cases; progressive disease (PD)—25% increase in size of one or more measurable lesions or the appearance of new lesions. Objective response (OR) was defined as CR + PR, and disease control (DC) as CR + PR + SD. ** Analysis of advanced patient subgroup: OR rate, P = 0.038; DC rate, P = 0.026; CR rate, P = 0.091 (chi-square analysis)
Supplementary analyses showed that in the 67% of patients with more advanced disease (Child Pugh B, ECOG 1, bilobar or recurrent disease), the incidence of OR and disease control rates were statistically higher (P = 0.038 and P = 0.026, respectively) in the DC Bead compared with the cTACE group (Fig. 2). The greatest difference in disease control rates between DC Bead and cTACE occurred in the ECOG 1 and Child-Pugh B subgroups (both, 63% and 32%, respectively; Fig. 3).
a Complete response, objective response, and disease control rate (cumulative number [%] of patients) of all patients at 6 months. b Complete response, objective response, and disease control rate (cumulative number [%] of patients) of patients by stratification factors for advanced disease at baseline
Safety
There was no statistically significant (P = 0.86) difference between treatments for the primary safety endpoint (treatment-related SAEs within 30 days of a procedure): 19 (20.4%) DC Bead patients experienced 28 events and 21 (19.4%) cTACE patients experienced 24 events. Supplementary post hoc analysis indicated that the incidence of SAEs within 30 days of a procedure was consistently lower in the DC Bead group both for the less advanced and for the more advanced patients based on the four stratification factors (Table 2).
The overall frequency of treatment-emergent AEs (TEAEs) per 100 treatments was lower in the DC Bead compared with the cTACE group, as were treatment-related TEAEs, Southwestern Oncology Group (SWOG) toxicity Grade 3 or 4 TEAEs, Grade 3 or 4 treatment-related TEAEs, and treatment-related SAEs. The majority of TEAEs were mild or moderate in intensity, with a lower frequency of severe events (20.4% vs. 30.6%) reported in DC Bead vs. cTACE patients. The only event with a difference in incidence of ≥10% was alopecia, reported in 2.2% of DC Bead and 19.4% of cTACE patients. Serious liver toxicity postchemoembolization was also lower in the DC Bead group. Observed postprocedural increases in the liver enzymes AST and ALT were significantly less in the DC Bead group than in the cTACE group. The mean maximum ALT increase in the DC Bead group was 50% less than in the cTACE group (95% CI, 39–65%; P < 0.001) and 41% less with respect to AST (95% CI, 46–76%; P < 0.001) (Fig. 4). Cardiac function (measured by echocardiography, isotopic ventriculography, or MRI) was maintained in the DC Bead group, whereas there was a deterioration in left ventricular ejection fraction in the cTACE group (DC Bead, +2.7 ± 10.1 percentage points; cTACE, −1.5 ± 7.6 percentage points; P = 0.018). Sixteen deaths were reported during the study (eight in each arm). Of these, two DC Bead and six cTACE patients died within 30 days of a procedure. Four patients died due to disease progression (one DC Bead, three TACE). Other causes of death were liver failure (two in each arm), cardiac events (two DC Bead, one cTACE), infection (one DC Bead, two cTACE), and, in one DC Bead patient, each of GI bleed and unknown cause.
Comparison of treatment groups for-fold changes in liver enzymes by chemoembolization procedure and maximum-fold change across all procedures (mean, 95% confidence interval [CI]). Analysis using t-test for log-transformed data; results back-transformed to ratio scale for presentation. Procedure 1B not shown due to small sample size. a Alanine aminotransferase (ALT): procedures 1 and 2 and maximum across all procedures, P < 0.001; procedure 3, P = 0.004. b Aspartate aminotransferase (AST): procedure 1, P = 0.001; procedure 2 and maximum across all procedures, P < 0.001; procedure 3, P = 0.06
With regard to the systemic side effects of doxorubicin (alopecia, skin discoloration, mucositis, and marrow suppression), post hoc analyses established a significant benefit (estimate of true incidence, −14.1%; 95% CI, −24.7% to –3.5%; P = 0.012) in favor of DC Bead over cTACE: 12 events in 11 (11.8%) patients vs. 40 events in 28 (25.9%) patients, respectively. Alopecia, the most commonly occurring event, was almost completely absent in DC Bead patients (1 vs. 23 events). The only DC Bead alopecia event was mild (Grade 1), while in the cTACE arm almost half of the alopecia events (11 events) were of pronounced/total hair loss (Grade 2). Marrow suppression and mucositis were more common and of greater severity in cTACE compared with DC Bead patients, and skin discoloration occurred in equal numbers (Table 3).Using the assumption of independence of events, the difference in frequencies of doxorubicin-related events was also significant (P = 0.0001). The incidence and frequency of post embolization syndrome events were comparable in the treatment groups: 35 events in 23 (24.7%) DC Bead and 43 events in 28 (25.9%) cTACE patients.
Discussion
Currently, cTACE is the standard first-line treatment for patients with inoperable and intermediate HCC. Although TACE has been in use for several years, the procedure varies widely between centers, with different drugs (doxorubicin, mitomycin, cisplatin, mixtures), embolic agents, doses, and schedules used. Response rates vary and evidence of a survival benefit, particularly at 3 years, is low [15–18]. In the PRECISION V study, cTACE was designed to reflect the current practice of chemoembolization and was standardized to the use of doxorubicin, lipiodol, and an embolic agent.
The DC Bead is a novel drug delivery embolization system that has been designed to deliver a higher and more sustained release of drug directly into the tumor and a low release of drug into the systemic circulation, with the intention to maximize the drug’s effectiveness in terms of response, while significantly reducing its systemic toxicity [22]. Preclinical and clinical studies have demonstrated a higher and prolonged retention of doxorubicin within the tumor after TACE with DC Bead, and lower systemic plasma levels of doxorubicin, compared to cTACE [19–24]. Varela et al. reported significantly lower doxorubicin Cmax and AUC values in DC Bead patients (78.97 ± 38.3 ng/ml and 662.6 ± 417.6 ng/ml min) than in cTACE patients (2341.5 ± 3951.9 ng/ml and 1812.2 ± 1093.7 ng/ml min; P = 0.00002 and P = 0.001, respectively)[23]. In another phase I/II clinical study Poon et al. observed low Cmax values (52.8 ± 41.5 ng/ml) even at the highest possible loading of doxorubicin (150 mg), with an average half-life of doxorubicin in plasma of 73.5 ± 22.7 h [24]. Clinical pilot studies in HCC have demonstrated promising efficacy [23–26, 33].
The current study is the first international, multicenter, randomized study designed to evaluate the safety and efficacy of a drug-eluting bead (DC Bead) compared to cTACE for the treatment of HCC. The 6-month OR rate of 52% observed following TACE with DC Bead in this study compares well with previously reported OR rates of 44–82% in phase I/II studies with DC Bead [23–26]. Although in this study statistical superiority in OR rates compared to cTACE could not be demonstrated, a trend toward higher response rates in all categories (complete response, OR, and disease control) was observed for DC Bead over cTACE. Of particular note was the significant reduction in serious liver toxicity and doxorubicin side effects with DC Bead, despite the higher mean total dose administered, enabling the physician to safely deliver higher doses of doxorubicin compared to cTACE.
Post hoc analyses of the predefined stratified groups of higher-risk patients demonstrated that DC Bead provided significant advantages in treating patients with more advanced disease, where improved response and disease control and good tolerability were achieved. This finding is of particular significance for Child-Pugh B and ECOG 1 patients, for whom treatment with chemoembolization has been controversial [6]. Contrary to the observation in the cTACE arm, the response rate for DC Bead in these subgroups was maintained. This suggests that the improved tolerability of DC Bead allows treatment to be repeated according to planned schedule even in these more vulnerable patients. These favorable results establish DC Bead as a viable embolic agent for studies evaluating the combination of TACE with systemic administration of targeted agents such as Sorafenib, in intermediate HCC. In advanced HCC, Sorafenib has shown a survival benefit with low radiological response rates [27–29].
A limitation of the current study is that the number of patients required to show statistically significant superiority was underestimated due to the higher response rate of cTACE (44%) compared with the original assumption (35%) [16]. Robust time-to-progression and survival data would require further randomized controlled trials with longer follow-ups.
In conclusion, TACE with DC Bead and doxorubicin is safe and effective in the treatment of intermediate-stage HCC and offers benefit to patients with more advanced disease.
References
Altekruse SF, McGlynn KA, Reichman ME (2009) Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27:1485–1491
Höpfner M, Schuppan D, Scherübl H (2008) Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol 14:1–14
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 19:329–338
Bruix J, Llovet JM (2002) Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35:519–524
O’Neil BH, Venook AP (2007) Hepatocellular carcinoma: the role of the North American GI Steering Committee Hepatobiliary Task Force and the advent of effective drug therapy. Oncologist 12:1425–1432
Bruix J, Sherman M (2005) Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Gish RG, Porta C, Lazar L et al (2007) Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 25:3069–3075
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Raoul JL, Heresbach D, Bretagne JF et al (1992) Chemoembolisation of hepatocellular carcinomas. A study of the biodistribution and pharmacokinetics of doxorubicin. Cancer 70:585–590
Lin DY, Liaw YF, Lee TY, Lai CM (1988) Hepatic arterial embolisation in patients with unresectable hepatocellular carcinoma—a randomised controlled trial. Gastroenterology 94:453–456
Group d’Etude et de Traitment du Carcinome Hépatocellulaire (1995) A comparison of lipiodol chemoembolisation and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 332:1256–1261
Raoul JL, Guyader D, Bretagne JF et al (1997) Prospective randomized trial of chemoembolisation versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 26:1156–1161
Bruix J, Llovet JM, Castells A et al (1998) Transarterial embolisation versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomised, controlled trial in a single institution. Hepatology 27:1578–1583
Pelletier G, Ducreux M, Gay F et al (1998) Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolisation: a multicentre randomised trial. J Hepatol 29:129–134
Lo CM, Ngan H, Tso WK et al (2002) Randomised controlled trial of transarterial lipiodol chemoembolisation for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739
Llovet JM, Bruix J, for the Barcelona-Clínic Liver Cancer Group (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolisation improves survival. Hepatology 37:429–442
Marelli L, Stigliano R, Triantos C et al (2007) Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Interv Radiol 30:6–25
Lewis AL, Gonzales MV, Lloyd AW et al (2006) DC Bead: in vitro characterisation of a drug-delivery device for transarterial chemoembolisation. J Vasc Interv Radiol 17:335–342
Lewis AL, Gonzalez MV, Leppard SW et al (2007) Doxorubicin eluting beads. 1. Effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med 18:1691–1699
Lewis AL, Taylor RR, Hall B et al (2006) Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolisation. J Vasc Interv Radiol 17:1335–1343
Hong K, Khwaja A, Liapi E et al (2006) New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 12:2563–2567
Varela M, Real MI, Burrel M et al (2007) Chemoembolisation of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 46:474–481
Poon RT, Tso WK, Pang RW et al (2007) A phase I/II trial of chemoembolisation for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 5:1100–1108
Kettenbach J, Stadler A, Katzler IV et al (2008) Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Interv Radiol 31:468–476
Malagari K, Chatzimichael K, Alexopöulou E et al (2008) Transarterial chemoembolisation of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Interv Radiol 31:269–280
Llovet JM, Di Bisceglie AM, Bruix J, Panel of Experts in HCC-Design Clinical Trials et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357:1191–1194
Hopewell S, Clarke M, Moher D et al (2008) CONSORT Group. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 371:281–283
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Wang SK, Tsiatis AA (1987) Approximately optional one-parameter boundaries for group sequential trials. Biometrics 43:193–199
Satake M, Uchida H, Arai Y et al (2008) Transcatheter arterial chemoembolization (TACE) with lipiodol to treat hepatocellular carcinoma: survey results from the TACE study group of Japan. Cardiovasc Interv Radiol 31:756–761
Grosso M, Vignali C, Quaretti P et al (2008) Transarterial chemoembolization of hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicenter study. Cardiovasc Interv Radiol 31:1141–1149
Acknowledgments
The study was sponsored by Biocompatibles UK Ltd. The authors acknowledge the contributions of Maria Schoder, Martin Funovics, Christian Loewe, Johanna Moyses, Dimitrios Kelekis, Nikolaos Kelekis, Alexis Kelekis, Emmanouil Emmanouil, Roman Klöckner, Christoph Düber, Gerd Otto, Marcus-Alexander Wörns, Verena Khan, Renate Hammerstingl, Clara Lee, Kathrin Eichler, Olivier Guillaud, Sabine Ficarelli, Nicolas Mennesson, Nathalie Rauscher, Beat Müllhaupt, Dominik Weishaupt, Pietro Majno, Laurent Spahr, Christoph D. Becker, Thierry de Baere, Frederic Deschamps, Pramod Roo, Vlad Ratzui, Phillipe Clluzel, Sebastien Novellas, Albert Tran, Denis Ouzan, Jean Gugenheim, Tim Greten, Timm Kirchhoff, Herbert Rosenthal, Joachim Lotz, Valerie Vilgrain, Annie Sibert, Peter Huppert, Hubertus Wietholtz, A Limmer, Pillippe Otal, Francis Joffre, Julien Auriol, Jean Marie Péron, Peter Waldenberger, Andreas Chemelli, Ivo Graziadei, Juergen Triller, and Ralph Kickuth (n = 52; order based on site recruitment).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The authors represent the PRECISION V Study Collaborators.
The detailed information about the PRECISION V Study collaborators are given in Appendix 1.
Appendix 1
Appendix 1
Austria
Medical University of Vienna
-
Department of Cardiovascular and Interventional Radiology: Johannes Lammer, Herbert Langenberger, Maria Schoder, Martin Funovics, Christian Loewe, Johanna Moyses
-
Department of Surgery: Thomas Grünberger
-
Department of Hepatology & Gastroenterology: Christian Müller
Medical University Innsbruck
-
Department of Radiology: Peter Waldenberger, Andreas Chemelli
-
Department of Gastroenterology & Hepatology: Ivo Graziadei
Germany
Goethe University, Frankfurt
-
Department of Radiology: Thomas Vogl, Verena Khan, Renate Hammerstingl, Clara Lee, Kathrin Eichler
-
Guttenberg University, Mainz
-
Department of Radiology: Michael Pitton, Roman Klöckner, Christoph Düber, Gerd Otto
-
Department of Internal Medicine: Marcus Schuchmann, Marcus-Alexander Wörns
-
Medizinische Hochschule Hannover
-
Tim Greten, Timm Kirchhoff, Herbert Rosenthal, Joachim Lotz
-
Klinikum Darmstadt
-
Department of Radiology: Peter Huppert
-
Department of Gastroenterology: Hubertus Wietholtz, A. Limmer
France
Hospices Civils de Lyon, CHU, Hopital Edouard Herriot
-
Department of Gastrointestinal Imaging: Frank Pilleul, Sabine Ficarelli, Nicolas Mennesson, Nathalie Rauscher
-
Department of Hepatogastroenterology: Jerome Dumortier, Olivier Guillaud
-
Hôpital Beaujon, Clichy
-
Valerie Vilgrain, Annie Sibert
-
Hôpital Archet II, Nice
-
Department of Radiology: Patrick Chevallier, Sebastien Novellas, Albert Tran, Denis Ouzan, Jean Gugenheim
-
Hôpital Huriez, Lille
-
Department of Radiology: Géraldine Sergent
CHU Rangueil, Toulouse
-
Department of Radiology: Pillippe Otal, Francis Joffre, Julien Auriol
Hôpital Purpan
-
Service d’Hépato-Gastro-Entérologie: Jean Marie Péron
Hôpital La Pitiè Salpetrière, Paris
-
Service d’Hépato-Gastroentérologie: Yves Benhamou, Vlad Ratzui, Phillipe Clluzel
Institut Gustave Roussy, Villejuif
-
Department of Radiology: Thierry de Baere, Frederic Deschamps, Pramod Roo
Hôpital Paul Brousse, Paris
-
Yves Ajavon, Sameh Awad, Marie-France Bellin, Didier Samuel, Denis Castaing, Rene Adam, Jean-Charles Duclos Vallee, Faouzy Saliba, Daniel Azoulay
Switzerland
CHU Vaudois, Lausanne
-
Department of Radiology: Alban Denys
Inselspital, Bern
-
Department of Radiology: Juergen Triller, Ralph Kickuth
Hopitaux Universitaires de Geneve
-
Department of Radiology: Sylvain Terraz, Christoph D. Becker
-
Department of Surgery: Pietro Majno
-
Department of Gastroenterology and Hepatology: Laurent Spahr
Universitaetsspital Zuerich
-
Department of Radiology: Thomas Pfammatter, Dominik Weishaupt
-
Clinic of Gastroenterology & Hepatology: Beat Muellhaupt
Greece
Eugenidion Hospital, University of Athens
-
Second Department of Radiology: Katerina Malagari, Pomoni Maria, Dimitrios Kelekis, Nikolaos Kelekis, Alexis Kelekis, Emmanouil Emmanouil
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lammer, J., Malagari, K., Vogl, T. et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc Intervent Radiol 33, 41–52 (2010). https://doi.org/10.1007/s00270-009-9711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9711-7