Abstract
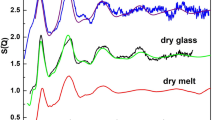
The structure of liquid silicates is commonly described as a statistical mixture of various atomic entities with relative abundances that can vary with pressure, temperature, and composition. Unfortunately, this view remains largely theoretical due to scarce experimental reports on the silicate melt structure, in particular under pressure. We performed X-ray diffraction of the SiO2 end member to probe the melting curve up to ~ 120 GPa and 7000 K, and the melt structure up to ~ 80 GPa. We confirm the steep increase of the melting curve above ~ 14 GPa when stishovite becomes stable over coesite in subsolidus conditions, with a slope of about 80 K/GPa. Then, around 45 GPa and 5400 K, the melting curve flattens significantly, an effect most likely reflecting the densification of the SiO2 melt structure. The signal of diffuse X-ray scattering is compatible with a change of the Si coordination number from 4 to 6 along the melting curve, in agreement with previous works reporting a similar evolution during the cold compression of SiO2 glass. Because of the limited pressure range (within 10 to 20 GPa) in which the melting curve changes its slope, we speculate a difficult coexistence of tetrahedral SiO4 and octahedral SiO6 units in SiO2 melt at high pressures.








Similar content being viewed by others
References
Akaogi M, Oohata M, Kojitani H, Kawaji H (2011) Thermodynamic properties of stishovite by low-temperature heat capacity measurements and the coesite–stishovite transition boundary. Am Mineral 96:1325–1330
Akins JA, Ahrens TJ (2002) Dynamic compression of SiO2: a new interpretation. Geophys Res Lett 29:1394
Andrault D, Fiquet G, Guyot F, Hanfland M (1998) Pressure-induced Landau-type transition in stishovite. Science 23:720–724
Andrault D, Angel RJ, Mosenfelder JL, Le Bihan T (2003) Equation of state of stishovite to lower mantle pressures. Am Mineral 88:301–307
Andrault D, Pesce G, Bouhifd MA, Bolfan-Casanova N, Henot JM, Mezouar M (2014) Melting of subducted basalt at the core-mantle boundary. Science 344:892–895
Belonoshko AB, Dubrovinsky LS (1995) Molecular-dynamics of stishovite melting. Geochim Cosmochim Acta 59:1883–1889
Benmore CJ, Soignard E, Amin SA, Guthrie M, Shastri SD, Lee PL, Yarger JL (2010) Structural and topological changes in silica glass at pressure. Phys Rev B. https://doi.org/10.1103/PhysRevB.81.054105
Brazhkin VV, Lyapin AG, Trachenko K (2011) Atomistic modeling of multiple amorphous–amorphous transitions in SiO2 and GeO2 glasses at megabar pressures. Phys Rev B. https://doi.org/10.1103/PhysRevB.83.132103
De Nolf W, Vanmeert F, Janssens K (2014) XRDUA: crystalline phase distribution maps by two-dimensional scanning and tomographic (micro) X-ray powder diffraction. J Appl Crystallogr 47:1107–1117. https://doi.org/10.1107/S1600576714008218
Dewaele A, Belonoshko AB, Garbarino G, Occelli F, Bouvier P, Hanfland M, Mezouar M (2012) High-pressure high-temperature equation of state of KCl and KBr. Phys Rev B. https://doi.org/10.1103/PhysRevB.85.214105
Fischer RA et al (2018) Equations of state and phase boundary for stishovite and CaCl2-type SiO2. Am Mineral 103:792–802. https://doi.org/10.2138/am-2018-6267
Geballe ZM, Jeanloz R (2012) Origin of temperature plateaus in laser-heated diamond anvil cell experiments. J Appl Phys 111:123518
Inamura Y, Katayama Y, Utsumi W, Funakoshi K (2004) Transformations in the intermediate-range structure of SiO2 glass under high pressure and temperature. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.93.015501
Lin JF et al (2007) Electronic bonding transition in compressed SiO2 glass. Phys Rev B 75:012201
Luo SN, Cagin T, Strachan A, Goddard WA, Ahrens TJ (2002) Molecular dynamics modeling of stishovite. Earth Planet Sci Lett 202:147–157
Meade C, Hemley RJ, Mao HK (1992) High-pressure X-ray diffraction of SiO2 glass. Phys Rev Lett 69:1387–1390
Murakami M, Bass JD (2010) Spectroscopic evidence for ultrahigh-pressure polymorphism in SiO2 glass. Phys Rev Lett 104:025504
Petitgirard S et al (2017) SiO2 glass density to lower-mantle pressures. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.119.215701
Prescher C, Prakapenka VB (2015) DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press Res 35:223–230
Prescher C, Prakapenka VB, Stefanski J, Jahn S, Skinner LB, Wang YB (2017) Beyond sixfold coordinated Si in SiO2 glass at ultrahigh pressures. Proc Natl Acad Sci USA 114:10041–10046
San LT, Hong NV, Hung PK (2016) Polyamorphism of liquid silica under compression based on five order-parameters and two-state model: a completed and unified description. High Press Res 36:187–197
Sanloup C et al (2013) Structural change in molten basalt at deep mantle conditions. Nature 503:104
Sato T, Funamori N (2010) High-pressure structural transformation of SiO2 glass up to 100 GPa. Phys Rev B 82:184102
Schultz E et al (2005) Double-sided laser heating system for in situ high pressure–high temperature monochromatic X-ray diffraction at the ESRF. High Press Res 25:71–83
Shen G, Lazor P (1995) Measurement of melting temperatures of some minerals under lower mantle conditions. J Geophys Res 100:17699–17713
Stixrude L, Karki BB (2005) Structure and freezing of MgSiO3 liquid in the Earth’s lower mantle. Science 310:297–299
Takada A, Bell RG, Catlow CRA (2016) Molecular dynamics study of liquid silica under high pressure. J Non-Cryst Solids 451:124–130
Usui Y, Tsuchiya T (2010) Ab initio two-phase molecular dynamics on the melting curve of SiO2. J Earth Sci 21:801–810
Wang FL, Tange Y, Irifune T, Funakoshi K (2012) P-V–T equation of state of stishovite up to mid-lower mantle conditions. J Geophys Res Solid Earth 117:B06209
Weck G, Garbarino G, Ninet S, Spaulding D, Datchi F, Loubeyre P, Mezouar M (2013) Use of a multichannel collimator for structural investigation of low-Z dense liquids in a diamond anvil cell: validation on fluid H2 up to 5 GPa. Rev Sci Instrum 84:063901
Wu M, Liang Y, Jiang J-Z, Tse JS (2012) Structure and properties of dense silica glass. Sci Rep 2:398
Zhang JZ, Liebermann RC, Gasparik T, Herzberg CT, Fei YW (1993) Melting and subsolidus relations of SiO2 at 9–14 GPa. J Geophys Res Solid Earth 98:19785–19793
Acknowledgements
We thank anonymous reviewers for helpful comments. This research was financed by the French Government Laboratory of Excellence Initiative n°ANR-10-LABX-0006, the Région Auvergne and the European Regional Development Fund. This is Laboratory of Excellence ClerVolc contribution N°384.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrault, D., Morard, G., Garbarino, G. et al. Melting behavior of SiO2 up to 120 GPa. Phys Chem Minerals 47, 10 (2020). https://doi.org/10.1007/s00269-019-01077-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-019-01077-3