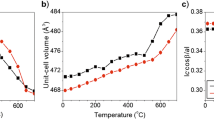
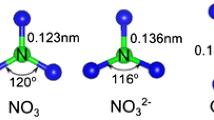
Abstract
We present experimental and calculated Al K-edge X-ray absorption near-edge structure (XANES) spectra of aluminous goethite with 10–33 mol% of AlOOH and diaspore. Significant changes are observed experimentally in the near- and pre-edge regions with increasing Al concentration in goethite. First-principles calculations based on density functional theory (DFT) reproduce successfully the experimental trends. This permits to identify the electronic and structural parameters controlling the spectral features and to improve our knowledge of the local environment of \(\hbox {Al}^{3+}\) in the goethite–diaspore partial solid solution. In the near-edge region, the larger peak spacing in diaspore compared to Al-bearing goethite is related to the nature (Fe or Al) of the first cation neighbours around the absorbing Al atom (Al*). The intensity ratio of the two near-edge peaks, which decreases with Al concentration, is correlated with the average distance of the first cations around Al* and the distortion of the \(\hbox {AlO}_6\) octahedron. Finally, the decrease in intensity of the pre-edge features with increasing Al concentration is due to the smaller number of Fe atoms in the local environment of Al since Al atoms tend to cluster. In addition, it is found that the pre-edge features of the Al K-edge XANES spectra enable to probe indirectly empty 3d states of Fe. Energetic, structural and spectroscopic results suggest that for Al concentrations around 10 mol%, Al atoms can be considered as isolated, whereas above 25 mol%, Al clusters are more likely to occur.






Similar content being viewed by others
References
Alvarez M, Rueda EH, Sileo E (2007) Simultaneous incorporation of Mn and Al in the goethite structure. Geochim Cosmochim Acta 71:1009–1020
Arrio MA, Rossano S, Brouder C, Galoisy L, Calas G (2000) Calculation of multipole transitions at the Fe \(K\) pre-edge through \(p-d\) hybridization in the Ligand Field Multiplet model. Europhys Lett 51:454–460
Bazilevskaya E, Archibald D, Aryanpour M, Kubicki J, Martínez C (2011) Aluminum coprecipitates with Fe (hydr)oxides: does isomorphous substitution of \(\text{ Al }^{\text{3+ }}\) for \(\text{ Fe }^{\text{3+ }}\) in goethite occur? Geochim Cosmochim Acta 75:4667–4683
Blanch AJ, Quinton JS, Lenehan CE, Pring A (2008) The crystal chemistry of Al-bearing goethites: an infrared spectroscopic study. Miner Mag 72:1043–1056
Blanchard M, Balan E, Giura P, Béneut K, Yi H, Morin G, Pinilla C, Lazzeri M, Floris A (2014) Infrared spectroscopic properties of goethite: anharmonic broadening, long-range electrostatic effects and Al substitution. Phys Chem Miner 41:289–302
Brouder C, Cabaret D, Juhin A, Sainctavit P (2010) Effect of atomic vibrations on the x-ray absorption spectra at the \(K\) edge of Al in \(\alpha {\text{-Al }}_{2}{\text{ O }}_{3}\) and of Ti in \({\text{ TiO }}_{2}\) rutile. Phys Rev B 81(115):125
Cabaret D, Brouder C (2009) Origin of the pre-edge structure at the Al \(K\)-edge: the role of atomic vibrations. J Phys Conf Ser 190(012):003
Cabaret D, Gaudry E, Taillefumier M, Sainctavit P, Mauri F (2005) XANES calculation with an efficient “non muffin-tin” method: application to the angular dependence of the Al K-edge in corundum. Phys Scr T115:131–133
Cornell R, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley, Weinheim
de Groot F, Grioni M, Fuggle J, Ghijsen J, Sawatzky G, Petersen H (1989) Oxygen 1s x-ray-absorption edges of transition-metal oxides. Phys Rev B 40:5715–5723
Farnan I, Balan E, Pickard C, Mauri F (2003) The effect of radiation damage on local structure in crystalline ZrSiO\(_4\): investigating the \(^{29}\)Si NMR response to pressure in zircon and reidite. Am Miner 88:1663–1668
Flank AM, Cauchon G, Lagarde P, Bac S, Janousch M, Wetter R, Dubuisson JM, Idir M, Langlois F, Moreno T, Vantelon D (2006) LUCIA, a microfocus soft XAS beamline. Nucl Instrum Methods Phys Res B 246:269–274
Fleet M (1976) Distortion parameters for coordination polyhedra. Miner Mag 40:531–533
Fleisch J, Grimm R, Gruübler J, Gütlich P (1980) Determination of the aluminum content of natural and synthetic alumogoethites using Mössbauer spectroscopy. J Phys 41:C1–169
Fritsch E, Morin G, Bedidi A, Bonnin D, Balan E, Caquineau S, Calas G (2005) Transformation of haematite and Al-poor goethite to Al-rich goethite and associated yellowing in a ferralitic clay soil profile of the middle Amazon Basin (Manaus, Brazil). Eur J Soil Sci 56:575–588
Garrity K, Bennett J, Rabe K, Vanderbilt D (2014) Pseudopotentials for high-throughput DFT calculations. Comput Mater Sci 81:446–452
Gaudry E, Cabaret D, Sainctavit P, Brouder C, Mauri F, Goulon J, Rogalev A (2005) Structural relaxations around Ti, Cr and Fe impurities in \(\alpha\)-Al\(_2\)O\(_3\) probed by x-ray absorption near edge structure combined with first-principles calculations. J Phys Condens Matter 17:5467–5480
Gaudry E, Cabaret D, Brouder C, Letard I, Rogalev A, Wilhlem F, Jaouen N, Sainctavit P (2007) Relaxations around the substitutional chromium site in emerald: X-ray absorption experiments and density functional calculations. Phys Rev B 76(094):110
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21(395):502
Gilbert B, Kim CK, Dong CL, Guo J, Nico PS, Shuh DK (2007) Oxygen K-edge emission and absorption spectroscopy in iron oxyhydroxide nanoparticles. AIP Conf Proc 882:721–725 proc. of the XAFS-13 conference, Stanford, USA, July 2006
Goodman B, Lewis D (1981) Mössbauer spectra of aluminous goethites. J Soil Sci 32:351–364
Gougoussis C, Calandra M, Seitsonen A, Mauri F (2009) First-principles calculations of X-ray absorption in a scheme based on ultrasoft pseudopotentials: From \(\alpha\)-quartz to high-Tc compounds. Phys Rev B 80(075):102
Ildefonse P, Cabaret D, Sainctavit P (1998) Aluminium X-ray absorption near edge structure in model compounds and Earth’s surface minerals. Phys Chem Miner 25:112–121
Kas JJ, Sorini AP, Prange MP, Cambell LW, Soininen JA, Rehr JJ (2007) Many-pole model of inelastic losses in X-ray absorption spectra. Phys Rev B 76(195):116
Kas JJ, Vinson J, Trcera N, Cabaret D, Shirley EL, Rehr JJ (2009) Many-pole model of inelastic losses applied to calculations of XANES. J Phys Conf Ser 190:012,009 proc. of the XAFS-14 conference, Camerino, Italie, July 2009
Kaur N, Gräfe M, Singh B, Kennedy B (2009) Simultaneous incorporation of Cr, Zn, Cd, and Pb in the goethite structure. Clays Clay Miner 57:234–250
Kim J, Ilott AJ, Middlemiss DS, Chernova NA, Pinney N, Morgan D, Grey CP (2015) \(^2\)H and \(^{27}\)Al solid-state NMR study of the local environments in Al-doped 2-line ferrihydrite, goethite, and lepidocrocite. Chem Mater 27:3966–3978
König E, Kremer S (1977) Ligand field energy diagrams. Plenum Publishing Corporation, New York
Lelong G, Radtke G, Cormier L, Bricha H, Rueff JP, Ablett JM, Cabaret D, Glbart F, Shukla A (2014) Detecting non-bridging oxygens: non-resonant inelastic X-ray scattering in crystalline lithium borates. Inorg Chem 53:10,903–10,908
Liu Q, Yu Y, Torrent J, Roberts A, Pan Y, Zhu R (2006) Characteristic low-temperature magnetic properties of aluminous goethite explained. J Geophys Res 111:B12S34
Manuel D, Cabaret D, Brouder C, Sainctavit P, Bordage A, Trcera N (2012) Experimental evidence of thermal fluctuations on the X-ray absorption near-edge structure at the aluminum \(K\) edge. Phys Rev B 85(224):108
Mitchell MR, Reader SW, Johnston KE, Pickard CJ, Whittle KR, Ashbrook SE (2011) \(^{119}\)Sn MAS NMR and first-principles calculations for the investigation of disorder in stannate pyrochlores. Phys Chem Chem Phys 13:488–497
Monkhorst H, Pack J (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:1227–1230
Murad E, Schwertmann U (1983) The influence of aluminum substitution and crystallinity on the Mössbauer-spectra of goethite. Clay Miner 18:301–312
Otte K, Pentcheva R, Schmahl WW, Rustad J (2009) Pressure-induced structural and electronic transitions in FeOOH from first principles. Phys Rev B 80(205):116
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Russell B, Payne M, Ciacchi L (2009) Density functional theory study of Fe(II) adsorption and oxidation on goethite surfaces. Phys Rev B 79(165):101
Scheinost A, Schulze DG, Schwertmann U (1999) Diffuse reflectance spectra of Al substituted goethite: a ligand field approach. Clays Clay Miner 47:156–164
Schulze DG (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays Clay Miner 32:36–44
Schulze DG, Schwertmann U (1987) The influence of aluminium on iron oxides: XII. Properties of goethites synthesised in \(0\cdot 3\) M KOH at \(25^{\circ }\text{ C }\). Clay Miner 22:83–92
Schwertmann U (1984) The influence of aluminium on iron oxides: IX. Dissolution of Al-goethites in 6 M HCl. Clays Miner 19:9–19
Sherman D, Waite T (1985) Electronic spectra of \(\text{ Fe }^{3+}\) oxides and oxide hydroxides in the near IR to near UV. Am Mineral 70:1262–1269
Taillefumier M, Cabaret D, Flank AM, Mauri F (2002) X-ray absorption near-edge structure calculations with the pseudopotentials: application to the K edge in diamond and \(\alpha\)-quartz. Phys Rev B 66(19):195,107
Trcera N, Cabaret D, Rossano S, Farges F, Flank AM, Lagarde P (2009) Experimental and theoretical study of the structural environment of magnesium in minerals and silicate glasses using x-ray absorption near-edge structure. Phys Chem Miner 36:241–257
Trivedi P, Axe L, Dyer J (2001) Adsorption of metal ions onto goethite: single-adsorbate and competitive systems. Colloids Surf A 191:107–121
Wang S, Mao W, Sorini A, Chen CC, Devereaux T, Ding Y, Xiao Y, Chow P, Hiraoka N, Ishii H, Cai Y, Kao CC (2010) High-pressure evolution of \({\text{ Fe }}_{2}{\text{ O }}_{3}\) electronic structure revealed by x-ray absorption. Phys Rev B 82(144):428
Wells MA, Fitzpatrick R, Gilkes R (2006) Thermal and mineral properties of Al-, Cr-, Mn-, Ni- and Ti-substituted goethite. Clays Clay Miner 54:176–194
Zhang J, Wu Z, Ibrahim K, Abbas M, Ju X (2003) Surface structure of \(\alpha\)-Fe2O3 nanocrystal observed by O \(K\)-edge X-ray absorption spectroscopy. Nucl Instrum Methods Phys Res B 199:291–294
Acknowledgments
We acknowledge SOLEIL for provision of synchrotron radiation facilities on the LUCIA beamline (project no: 99140120). This work was performed using HPC resources from GENCI-IDRIS (Grant 2014 - 100172). This work has been supported by the French National Research Agency (ANR, project 11-JS56-001 “CrIMin”). The authors thank Blair Lebert for proofreading the manuscript. Etienne Balan and Amélie Juhin are acknowledged for fruitful discussions. DC dedicates this paper to the memory of her late colleague, Philippe Ildefonse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ducher, M., Blanchard, M., Vantelon, D. et al. Probing the local environment of substitutional Al\(^{3+}\) in goethite using X-ray absorption spectroscopy and first-principles calculations. Phys Chem Minerals 43, 217–227 (2016). https://doi.org/10.1007/s00269-015-0788-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-015-0788-z