Abstract
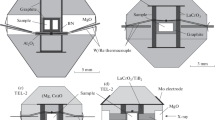
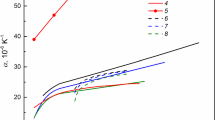
Magnesium carbonate MgCO3 (magnesite) was experimentally studied at pressures of 12–84 GPa and temperatures between 1,600 and 3,300 K. We applied the high-pressure technique using a multianvil press and a diamond anvil cell with laser heating. The phase relations and melting of magnesite were investigated by means of Raman and time-resolved multi-wavelength spectroscopy. Magnesite is found to melt congruently within the entire studied pressure range at temperatures of 2,100–2,650 K. At temperatures above 2,700 K, we observed decomposition of magnesite with formation of MgO and a carbon phase (diamond). Our results demonstrate that at high pressures, the magnesium carbonate melt can exist at a wide range of thermodynamic conditions.








Similar content being viewed by others
References
Akahama Y, Kawamura H (2006) Pressure calibration of diamond anvil Raman gauge to 310 GPa. J Appl Phys 100:043516. doi:10.1063/1.2335683
Biellmann C, Gillet P, Guyot F et al (1993) Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet Sci Lett 118:31–41. doi:10.1016/0012-821X(93)90157-5
Boulard E, Gloter A, Corgne A et al (2011) New host for carbon in the deep Earth. 108:5184–5187. doi:10.1073/pnas.1016934108
Boulard E, Menguy N, Auzende AL et al (2012) Experimental investigation of the stability of Fe-rich carbonates in the lower mantle. J Geophys Res 117:B02208. doi:10.1029/2011JB008733
Brenker FE, Vollmer C, Vincze L et al (2007) Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet Sci Lett 260:1–9. doi:10.1016/j.epsl.2007.02.038
Bulanova GP, Pavlova LP (1987) Magnesite peridotite mineral association in a diamond from the Mir pipe. Dokl Akad Nauk SSSR 295:1452–1456
Bundy FP, Bassett WA, Weathers MS et al (1996) The pressure-temperature phase and transformation diagram for carbon; updated through 1994. Carbon N Y 34:141–153. doi:10.1016/0008-6223(96)00170-4
Dasgupta R, Hirschmann MM (2010) The deep carbon cycle and melting in Earth’s interior. Earth Planet Sci Lett 298:1–13. doi:10.1016/j.epsl.2010.06.039
Dubrovinskaia N, Eska G, Sheshin GA, Braun H (2006) Superconductivity in polycrystalline boron-doped diamond synthesized at 20 GPa and 2700 K. J Appl Phys 99:033903. doi:10.1063/1.2166645
Dubrovinsky L, Glazyrin K, McCammon C et al (2009) Portable laser-heating system for diamond anvil cells. J Synchrotron Radiat 16:737–741. doi:10.1107/S0909049509039065
Fiquet G, Guyot F, Kunz M et al (2002) Structural refinements of magnesite at very high pressure. Am Mineral 87:1261–1265
Frost D, Poe B, Tronnes R et al (2004) A new large-volume multianvil system. Phys Earth Planet Inter 143–144:507–514. doi:10.1016/j.pepi.2004.03.003
Gillet P, Biellmann C, Reynard B, McMillan P (1993) Raman spectroscopic studies of carbonates part I: high-pressure and high-temperature behavior of calcite, magnesite, dolomite and aragonite. Phys Chem Miner. doi:10.1007/BF00202245
Hanfland M, Syassen K (1985) A Raman study of diamond anvils under stress. J Appl Phys 57:2752. doi:10.1063/1.335417
Irving AJ, Wyllie PJ (1973) Melting relationships in CaO-CO2 and MgO-CO2 to 36 kilobars with comments on CO2 in the mantle. Earth Planet Sci Lett 20:220–225. doi:10.1016/0012-821X(73)90161-1
Isshiki M, Irifune T, Hirose K et al (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427:60–63. doi:10.1038/nature02181
Ito E, Katsura T (1992) High pressure research: application to earth and planetary sciences. Geophys Monogr Ser 67:315–322. doi:10.1029/GM067
Kaminsky FV, Wirth R, Schreiber A (2014) Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: new minerals in the carbonate-halide association. Can Mineral 51:669–688. doi:10.3749/canmin.51.5.669
Katsura T, Ito E (1990) Melting and subsolidus phase relations in the MgSiO3–MgCO3 system at high pressures: implications to evolution of the Earth’s atmosphere. Earth Planet Sci Lett 99:110–117. doi:10.1016/0012-821X(90)90074-8
Katsura T, Yoneda A, Yamazaki D et al (2010) Adiabatic temperature profile in the mantle. Phys Earth Planet Inter 183:212–218. doi:10.1016/j.pepi.2010.07.001
Kupenko I, Dubrovinsky L, Dubrovinskaia N et al (2012) Portable double-sided laser-heating system for Mössbauer spectroscopy and X-ray diffraction experiments at synchrotron facilities with diamond anvil cells. Rev Sci Instrum 83:124501. doi:10.1063/1.4772458
Litasov KD, Goncharov AF, Hemley RJ (2011) Crossover from melting to dissociation of CO2 under pressure: implications for the lower mantle. Earth Planet Sci Lett 309:318–323. doi:10.1016/j.epsl.2011.07.006
Litvin YuA, Vasiliev PG, Bobrov AV et al (2011) Parental media for diamonds and primary inclusions by evidence of physicochemical experiment. Vestn Otd Nauk o Zemle RAN 3:1–7. doi:10.2205/2011NZ000196
Litvin Yu, Spivak A, Solopova N, Dubrovinsky L (2014) On origin of lower-mantle diamonds and their primary inclusions. Phys Earth Planet Inter. doi:10.1016/j.pepi.2013.12.007
Oganov AR, Ono S, Ma Y et al (2008) Novel high-pressure structures of MgCO3, CaCO3 and CO2 and their role in Earth’s lower mantle. Earth Planet Sci Lett 273:38–47. doi:10.1016/j.epsl.2008.06.005
Pippinger T, Dubrovinsky L, Glazyrin K et al (2011) Detection of melting by in situ observation of spherical-drop formation in laser-heated diamond-anvil cells 23:29–41
Prakapenka VB, Kubo A, Kuznetsov A et al (2008) Advanced flat top laser heating system for high pressure research at GSECARS: application to the melting behavior of germanium. High Press Res 28:225–235. doi:10.1080/08957950802050718
Scott HP, Doczy VM, Frank MR et al (2013) Magnesite formation from MgO and CO2 at the pressures and temperatures of Earth’s mantle. Am Mineral 98:1211–1218. doi:10.2138/am.2013.4260
Shen G, Wang L, Ferry R et al (2010) A portable laser heating microscope for high pressure research. J Phys Conf Ser 215:012191. doi:10.1088/1742-6596/215/1/012191
Skorodumova NV (2005) Stability of the MgCO3 structures under lower mantle conditions. Am Mineral 90:1008–1011. doi:10.2138/am.2005.1685
Solopova NA, Litvin YuA, Spivak AV et al (2013) The phase diagram of Na carbonate, an alkaline component of the growth medium of ultradeep diamonds. Dokl Earth Sci 453:1106–1109. doi:10.1134/S1028334X13110068
Spivak AV, Litvin YuA, Ovsyannikov SV et al (2012) Stability and breakdown of Ca13CO3 melt associated with formation of 13C-diamond in static high pressure experiments up to 43 GPa and 3900K. J Solid State Chem 191:102–106. doi:10.1016/j.jssc.2012.02.041
Stagno V, Tange Y, Miyajima N, et al (2011) The stability of magnesite in the transition zone and the lower mantle as function of oxygen fugacity. Geophys Res Lett 38. doi: 10.1029/2011GL049560
Tao R, Fei Y, Zhang L (2013) Experimental determination of siderite stability at high pressure. Am Mineral 98:1565–1572. doi:10.2138/am.2013.4351
Tschauner O, Mao H, Hemley R (2001) New transformations of CO2 at high pressures and temperatures. Phys Rev Lett 87:075701. doi:10.1103/PhysRevLett.87.075701
Acknowledgments
The study was financially supported by the German Research Foundation (DFG) and the Russian Foundation for Basic Research (Nos. 13-05-00835, 14-05-31142 and 11-05-00401). N.D. thanks DFG for financial support through the Heisenberg Program and the DFG Project DU 954-8/1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solopova, N.A., Dubrovinsky, L., Spivak, A.V. et al. Melting and decomposition of MgCO3 at pressures up to 84 GPa. Phys Chem Minerals 42, 73–81 (2015). https://doi.org/10.1007/s00269-014-0701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-014-0701-1