Abstract
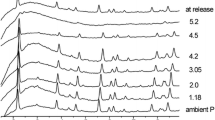
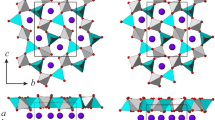
Synchrotron single-crystal X-ray diffraction experiments at high-pressure and high-temperature conditions were performed up to 20 GPa and 573.0(2) K on a fully ordered stoichiometric dolomite and a partially disordered stoichiometric dolomite [order parameter, s = 0.26(6)]. The ordered dolomite was found to be stable up to approximately 14 GPa at ambient temperature and up to approximately 17 GPa at T = 573.0(2) K. The P–V data from the ambient temperature experiments were analysed by a second-order Birch–Murnaghan equation-of-state giving K 0 = 92.7(9) GPa for the ordered dolomite and K 0 = 92.5(8) GPa for the disordered dolomite. The high-temperature data, collected for the ordered sample, were fitted by a third-order Birch–Murnaghan equation-of-state resulting in K 0 = 95(6) GPa and K′ = 2.6(7). In order to compare the three experiments results, a third-order Birch–Murnaghan equation-of-state was also calculated for the ambient temperature experiments giving K 0 = 93(3) GPa, K′ = 3.9(6) for the ordered dolomite and K 0 = 92(3) GPa, K′ = 4.0(4) for the disordered dolomite. The derived axial moduli show that dolomite compresses very anisotropically, being the c-axis approximately three times more compressible than the a-axis. The axial compressibility increases as T increases, and the a-axis is the most temperature-influenced axis. On the contrary, axial compressibility is not influenced by disordering. Structural refinements at different pressures show that Ca and Mg octahedra are almost equally compressible in the ordered dolomite with K(CaO6) = 109(4) GPa and K(MgO6) = 103(3) GPa. On the contrary, CaO6 compressibility is reduced and MgO6 compressibility is increased in the disordered crystal structure where K(CaO6) = 139(4) GPa and K(MgO6) = 89(4) GPa. Disordering is found to increase CaO6 and to decrease MgO6 bond strengths, thus making stiffer the Ca octahedron and softer the Mg octahedron. Cation polyhedra are distorted in both ordered and disordered dolomites and they increase in regularity as P increases. Ordered dolomite approaches regularity at approximately 14 GPa. The increase in regularity of octahedra in the disordered dolomite is strongly affected by the very slow regularization of MgO6 with respect to CaO6. The phase transition to the high-pressure polymorph of dolomite (dolomite-II), which is driven by a significant increase in the regularity of both cations polyhedra and mineral crystal structure, occurs in the ordered dolomite at ambient temperature at approximately 14 GPa; whereas no clear evidences of phase transition were observed as regards the disordered crystal structure.









Similar content being viewed by others
References
Angel RJ (2000) Equation of state. In: R.M. Hazen and R.T. Downs (ed) High- temperature and High-pressure Crystal Chemistry, 41, pp. 35–59. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, Chantilly,Virginia
Angel RJ (2002) EOS-FIT5.2. Computer program. Crystallography Laboratory, Department of Geological Sciences, Virginia Tech, Blacksburg
Antao SM, Mulder WH, Hassan I, Crichton WA, Parise JB (2004) Cation disorder in dolomite, CaMg(CO3)2, and its influence on the aragonite + magnesite → dolomite reaction boundary. Am Miner 89:1142–1147
Bass JD, Liebermann RC, Weidner DJ, Finch SJ (1981) Elastic proper- ties from acoustic and volume compression experiments. Phys Earth Planet Inter 25:140–158
Birch F (1952) Elasticity and constitution of earth’s interior. J Geophys Res 57(2):227–286
Buob A, Luth RW, Schmidt MW, Ulmer P (2006) Experiments on CaCO3–MgCO3 solid solutions at high-pressure and temperature. Am Miner 91:435–440
Datchi F, LeToullec R, Loubeyre P (1997) Improved calibration of the SrB4O7:Sm2+ optical pressure gauge: advantages at very high pressures and high temperatures. J Appl Phys 81:3333–3339
Degtyareva O (2010) Simple Metals at High Pressures, In: Boldyreva, E, Dera, P (eds) High-pressure crystallography from fundamental phenomena to technological applications, series: NATO science for peace and security series B: physics and biophysics. pp 261–280
Fiquet G, Guyot F, Itie J-P (1994) High-pressure X-ray diffraction study of carbonates: MgCO3,·CaMg(CO3)2, and CaCO3. Am Miner 79:15–23
Forman RA, Piermarini GJ, Barnett JD, Block S (1972) Pressure measurement made by utilization of ruby sharp-line luminescence. Science 176:284–286
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D (1996) Two-dimensional detector software: from real detector to idealized image or two-theta scan. High Pressure Res 14:235–245
Hammouda T, Andrault D, Koga K, Katsura T, Martin AM (2011) Ordering in double carbonates and implications for processes at subduction zones. Contrib Miner Pet 161(3):439–450
Korsakov AV, Hermann J (2006) Silicate and carbonate melt inclusions associated with diamonds in deeply subducted carbonate rocks. Earth Planet Sci Lett 241:104–118
Lin JF, Liu J, Jacobs C, Prakapenka VB (2012) Vibrational and elastic properties of ferromagnesite across the electronic spin-pairing transition of iron. Am Miner 97:583–591
Luth RW (2001) Experimental determination of the reaction aragonite + magnesite = dolomite at 5–9 GPa. Contrib Miner Petr 141:222–232
Mao Z, Armentrout M, Rainey E, Manning CE, Dera P, Prakapenka VB, Kavner A (2011) Dolomite III: a new candidate lower mantel carbonate. Geophys Res Lett 38:L22303
Martinez I, Zhang J, Reeder RJ (1996) In situ X-ray diffraction of aragonite and dolomite at high-pressure and high-temperature; evidence for dolomite breakdown to aragonite and magnesite. Am Miner 81:611–624
Merlini M, Crichton WA, Hanfland M, Gemmi M, Müller H, Kupenko I, Dubrovinsky L (2012) Structures of dolomite at ultrahigh-pressure and their influence on the deep carbon cycle. Proc Natl Acad Sci USA 109(34):13509–13514
Parise JB, Antao SM, Martin CD, Crichton W (2005) Diffraction studies of order-disorder at high pressures and temperatures. Powder Diffr 20:80–86
Prewitt CT, Downs RT (1998) High-pressure crystal chemistry. Rev Miner 37:283–312
Raju SV, Knight J, Pawley AR, Clark SM (2008) Phase diagram determination up to 823 K in minerals using diamond anvil cell. AGU Fall Meeting Abstracts, 1, 1729
Reeder RJ (1983) Crystal chemistry of the rhombohedral carbonates. Rev Miner Geochem 11(1):1–47
Reeder RJ, Wenk HR (1983) Structure refinements of some thermally disordered dolomites. Am Miner 68:769–777
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Rodriguez-Navarro C, Kudlacz K, Ruiz-Agudo E (2012) The mechanism of thermal decomposition of dolomite: new insights from 2D-XRD and TEM analyses. Am Miner 97:38–51
Ross NL, Reeder RJ (1992) High-pressure structural study of dolomite and ankerite. Am Miner 77:412–421
Santillán J, Williams Q, Knittle E (2003) Dolomite-II: a high-pressure polymorph of CaMg(CO3)2. Geophys Res Lett 30(2):1054
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Thompson RM, Downs RT (2010) Packing systematics of the silica polymorphs: the role played by O–O nonbonded interactions in the compression of quarts. Am Miner 95:104–111
van Roermund HLM, Carswell DA, Drury MR, Heijboer TC (2002) Microdiamonds in a megacrystic garnet websterite pod from Bardane on the island of Fjortoft, western Norway: evidence for diamond formation in mantle rocks during deep continental subduction. Geology 30:959–962
Wang A, Pasteris JD, Meyer HOA, Dele-Duboi ML (1996) Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet Sc Lett 141:293–306
Wenk HR, Barber DJ, Reeder RJ (1983) Microstructures in carbonates. Rev Miner Geochem 11(1):301–367
Wilson AJC, Prince E (1999) International tables for crystallography volume C, vol 2. Kluwer, Dordrecht
Zhu Y, Ogasawara Y (2002) Carbon recycled into deep Earth: evidence from dolomite dissociation in subduction-zone rocks. Geology 30:947–950
Zucchini A, Comodi P, Katerinopoulou A, Balic-Zunic T, McCammon C, Frondini F (2012) Order–disorder–reorder process in thermally treated dolomite samples: a combined powder and single-crystal X-ray diffraction study. Phys Chem Miner 39(4):319–328
Acknowledgments
Mark Welch is thanked for kindly providing the dolomite sample from the Natural History Museum of London and for his detailed review of the manuscript. Wilson Crichton and the Editor Milan Rieder are also greatly acknowledged on reviewing the manuscript. The European Synchrotron Facility is acknowledged for allocating beam-time for the experiment. This study was partly supported by the research project PRIN-MIUR 2010-2011 to Paola Comodi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zucchini, A., Comodi, P., Nazzareni, S. et al. The effect of cation ordering and temperature on the high-pressure behaviour of dolomite. Phys Chem Minerals 41, 783–793 (2014). https://doi.org/10.1007/s00269-014-0691-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-014-0691-z