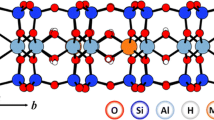
Abstract
We report studies based on a combination of ab initio electronic structure and Monte Carlo (MC) technique on the problem of cation partitioning among inequivalent octahedral sites, M1 and M2 in mixed olivines containing Mg2+ and Fe2+ ions. Our MC scheme uses interactions derived out of ab initio, density functional calculations carried out on measured crystal structure data. Our results show that there is no reversal of the preference of Fe for M1 over M2 as a function of temperature. Our findings do not agree with the experimental findings of Redfern et al. (Phys Chem Miner 27:630–637, 2000), but are in agreement with those of Heinemann et al. (Eur J Mineral 18:673–689, 2006) and Morozov et al. (Eur J Mineral 17:495–500, 2005).






Similar content being viewed by others
Notes
The order–disorder process in olivine is called non-convergent because M1 and M2 sites do not converge to symmetry equivalence even when Fe2+ and Mg2+ become randomly disordered.
Contribution for h vanishes for 50:50 Fe–Mg olivine.
The standard deviations are large for small quantities like J and J′ which are already at the borderline of DFT accuracy.
References
Abdu YA, Annersten H, Ericsson T, Hawthorne FC (2008) High-temperature cation ordering in olivine: an in situ Mössbauer study of synthetic (Mg0.55Fe0.45)2SiO4. Hyperfine Interact 186:99–103
Artioli G, Rinaldi R, Wilson CC, Zanazzi PF (1995) High-temperature Fe-Mg cation partitioning in olivine: in situ single-crystal neutron diffraction study. Am Mineral 80:197–200
Binder K, Heermann DW (2002) Monte Carlo simulation in statistical physics: an introduction. Springer, Berlin
Birle JD, Gibbs GV, Moore PB, Smith JV (1968) Crystal structure of natural olivines. Am Mineral 53:807–824
Burns RG (1970) Crystal field spectra and evidence of cation ordering in olivine minerals. Am mineral 55:1609
Bush W, Hafner SS, Virgo D (1970) Some ordering of iron and magnesium at octahedrally coordinated sites in a magnesium-rich olivine. Nature 227:1339
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474
Chatterjee S, Sengupta S, Saha-Dasgupta T, Chatterjee K, Mandal N (2009) Site preference of Fe atoms in FeMgSiO4 and FeMg(SiO3)2 studied by density functional calculations. Phys Rev B 79:115103
Chatterjee S, Saha-Dasgupta T (2010) First-Principles simulations of structural, electronic, and magnetic properties of vacancy bearing Fe silicates. Phys Rev B 81:155105
Finger LW (1970) Fe/Mg ordering in olivines. Carnegie Institution of Washington year book, vol 69, pp 302–305
Ganguly J, Cheng W, O’Neill HSTC (1993) Syntheses, volume, and structural changes of garnets in the pyrope-grossular join: Implications for stability and mixing properties. Am Miner 78:583–593
Ganguly J, Tazzoli V (1994) Fe2+-Mg interdiffusion in orthopyroxene: retrieval from the data on intracrystallline exchange reaction. Am Mineral 79:1053–1067
Ghose S, Wan C, McCallum IS (1976) Fe2+-Mg2+ order in an olivine from the lunar anorthosite 67075 and the significance of cation order in lunar and terrestrial olivines. Indian J Earth Sci 3:1
Heinemann R, Staack V, Fischer A, Kroll H, Thomas V, Kirfel A (1999) Temperature dependence of Fe, Mg partitioning in Acapulco olivine. Am Mineral 84:1400–1405
Heinemann R, Kroll H, Langenhorst F, Lueder T (2000) Time and temperature variation of the intracrystalline Fe2+, Mg fractionation in Johnstown meteoritic orthopyroxene. Eur J Mineral 12:163–176
Heinemann R, Kroll H, Kirfel A, Barbier B (2003) Die Temperaturabhängigkeit der Fe2+, Mg-Verteilung in Olivinen. Ber. Dtsch. Mineral. Ges, Beih. z. Eur J Mineral, 15, Bh.1, 78 (in German)
Heinemann R, Kroll H, Kirfel A, Barbier B (2006) Order and anti-order in olivine I: structural response to temperature. Eur J Mineral 18:673–689
Hubbard J (1963) Electron correlations in narrow energy bands. Proc R Soc Lond Ser A 276:238–257
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558
Kresse G, Furthmuller J (1996a) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15
Kresse G, Furthmuller J (1996b) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169
Kroll H, Knitter R (1991) Al, Si exchange kinetics in sanidine and anorthoclase and modeling of rock cooling paths. Am Mineral 76:928–941
Kroll H, Schlenz H, Phillips MW (1994) Thermodynamic modelling of non-convergent ordering in orthopyroxenes: a comparison of classical and Landau approaches. Phys Chem Miner 21:555–560
Morozov M, Brinkmann C, Lottermoser W, Tipplet G, Amthauer G, Kroll H (2005) Octahedral cation partitioning in Mg, Fe2+-olivine. Mössbauer spectroscopic study of synthetic (Mg0.5Fe2+ 0.5)2SiO4 (Fa50). Eur J Mineral 17:495–500
Putnis A (1992) An introduction to mineral sciences. Cambridge University Press, Cambridge
Redfern SAT, Artioli G, Rinaldi R, Henderson CMB, Knight KS, Wood BJ (2000) Octahedral cation ordering in olivine at high temperature. II: An in situ neutron powder diffraction study on synthetic MgFeSiO4 (Fa50). Phys Chem Miner 27:630–637
Rinaldi R, Wilson CC (1996) Crystal Dynamics by neutron time-of-flight Laue diffraction in olivine up to 1573 K using single frame methods. Solid State Commun 97:395–400
Rinaldi R, Artioli G, Wilson CC, McIntyre G (2000) Octahedral cation ordering in olivine at high temperature. I: In situ neutron single-crystal diffraction studies on natural mantle olivines (Fa12 and Fa10). Phys Chem Minerals 27:623–629
Seifert FA, Virgo D (1975) Kinetics of Fe2+-Mg order-disorder in anthophyllites: quantitative cooling rates. Science 188:1107–1109
Smyth JR, Hazen RM (1973) The crystal structures of forsterite and hortonolite at several temperatures up to 900°C. Am Mineral 58:588–593
Wenk HR, Raymond KN (1973) Four new structure refinements of olivine. Z Kristallogr 137:86–105
Acknowledgment
We thank funding through Advanced Materials Research Unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, S., Bhattacharyya, S., Sengupta, S. et al. Crossover of cation partitioning in olivines: a combination of ab initio and Monte Carlo study. Phys Chem Minerals 38, 259–265 (2011). https://doi.org/10.1007/s00269-010-0401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-010-0401-4