Abstract
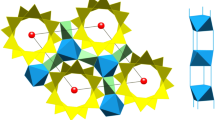
A number of different impurities are located in the open channels of natural beryl crystals. The rare Maxixe beryl contains an unusual amount of NO2. The isoelectronic CO2 − radical is found in the irradiated Maxixe-type beryl. The NO2 radicals are distributed in the Be–Al plane of the crystal, with the nitrogen atom close to the oxygens of the beryl cavity wall. These oxygens repel the negative CO2 − radical, which is located at the center of the beryl cavity and rotates around its O–O axis, which is parallel to the crystal c-axis. When there is a nearby alkali ion at the center of the beryl channel, it reorients the CO2 − radical so that its bisector is parallel to the c-axis and points toward the positive ion. Different signals are analyzed for Li+, Na+, and another counter-ion, which probably is Cs+. The related NO3 and CO3 − radicals are the color centers in the investigated deep blue beryls. The slow decay of the color, which makes these beryls useless as gem stones, is related to the decay of the hydrogen atoms which are present in these crystals. Evidence is given that NO3 is created in Maxixe beryl by a natural process, while CO3 − in Maxixe-type beryl has been created by irradiation. The temperature dependence of the EPR signals of these two radicals was investigated, but a definitive proof that they rotate at the center of the beryl cavity could not be given. EPR signals from some other radicals in beryl have been observed and described.












Similar content being viewed by others
References
Andersson LO (1976) Selective line cancellation in overlapping EPR spectra using 90° out-of-phase detection. Applications to Maxixe type beryl. Proceedings of the 19th Congress Ampere, p 535
Andersson LO (1979) The difference between Maxixe beryl and Maxixe-type beryl: an electron paramagnetic resonance investigation. J Gemm 16:313–317
Andersson LO (2006) The positions of H+, Li+ and Na+ impurities in beryl. Phys Chem Miner 33:403–416
Andersson LO (2008) EPR investigation of the methyl radical, the hydrogen atom and carbon oxide radicals in Maxixe-type beryl. Phys Chem Miner 33:403–416
Atkins PW, Symons MCR (1967) The structure of inorganic radicals. Elsevier, Amsterdam
Bentley J, Carmichael I (1985) Electron spin properties of complexes formed by Li or Na with CO2. J Phys Chem 89:4040–4042
Borel JP, Faes F, Pittet A (1981) Electron paramagnetic resonance of Li–CO2 complexes in a CO2 matrix at 77 K. J Chem Phys 74:2120–2123
Borisenko KB, Kolonits M, Rozsondai B, Hargittai I (1997) Electron diffraction study of the nitrogen dioxide molecular structure at 294, 480, and 691 K. J Mol Struct 413/414:121–131
Bragg WL, West J (1926) The structure of beryl Be3Al2Si6O18. Proc R Soc Lond Ser A 111:691–714
Brown GE, Mills BA (1986) High-temperature structure and crystal chemistry of hydrous alkali-rich beryl from Harding pegmatite, Taos County, New Mexico. Am Miner 71:547–556
Chantry GW, Horsfield A, Morton JR, Whiffen DH (1962) The structure, electron resonance and optical spectra of trapped CO3 − and NO3. Mol Phys 5:589–599
Dvir M, Low W (1960) Paramagnetic resonance and optical spectrum of iron in beryl. Phys Rev 119:1587–1591
Edgar A, Vance ER (1977) Electron paramagnetic resonance, optical absorption, and magnetic circular dichroism studies of the CO3 − molecular-ion in irradiated natural beryl. Phys Chem Miner 1:165–178
Eisfeld W, Morokuma K (2000) A detailed study on the symmetry breaking and its effect on the potential surface of NO3. J Chem Phys 113:5587–5597
Fujimura T, Iwasaki H, Sasaki S, Sha RZ (1994) Crystal structure of NaNO3 at high pressure. High Press Res 11:337–345
Gibbs GV, Breck DW, Meagher EP (1968) Structural refinement of hydrous and anhydrous synthetic beryl, Al2(Be3Si6)O18 and emerald, Al1.9Cr0.1(Be3Si6)O18. Lithos 1:275–285
Jordan KD (1984) Theoretical investigation of lithium and sodium complexes with CO2. J Phys Chem 88:2459–2465
Krambrock K, Pinheiro MVB, Guedes KJ, Medeiros SM, Schweizer S, Castaneda C, Botelho NF, Pedrosa-Soares AC (2002) Radiation-induced centers in Cs-rich beryl studied by magnetic resonance, infrared and optical spectroscopy. Nucl Instrum Methods B 191:285–290
Kusch P, Taub H (1949) On the g J values of the alkali atoms: the hyperfine structure of the alkali atoms. Phys Rev 75:1477–1480
McMillan JA, Marshall SA (1968) Motional effects in the electron spin resonance absorption spectrum of CO2 − molecule-ions in single-crystal calcite. J Chem Phys 48:467–471
Morosin B (1972) Structure and thermal expansion of beryl. Acta Crystallogr B28:1899–1903
Nassau K, Prescott BE, Wood DL (1976) The deep blue Maxixe-type color center in beryl. Am Miner 61:100–107
Ovenall DW, Whiffen DH (1961) Electron spin resonance and structure of the CO2 − radical ion. Mol Phys 4:135–144
Pinheiro MVB, Krambrock K, Guedes KJ, Spaeth JM (2007) Optically-detected magnetic resonance of molecular color centers CO3 − and NO3 in gamma-irradiated beryl. Phys Stat Sol C 4:1293–1296
Schaafsma TJ et al (1968) Electron spin resonance of NO2. I: the ESR spectrum of NO2 in a polycristalline matrix of N2O4. Mol Phys 14:501–515
Schlossmacher K, Klang H (1935) Der Maxixeberyll. I Zentralbl Mineral Geol Paläont 1935A:37–44
Schramm DU, Rossi AM (2000) EPR and ENDOR studies on CO2 − radicals in γ-irradiated B-type carbonated apatites. Phys Chem Chem Phys 2:1339–1343
Serway RA, Marshall SA (1967) Electron spin resonance absorption spectra of CO3 − and CO3 3− molecule-ions in irradiated single-crystal calcite. J Chem Phys 46:1949–1952
Solntsev VP (1981) The nature of color centers and EPR in beryl and chrysoberyl. Trudy Instituta Geologii i Geofiziki, Akademia Nauk SSSR, issue 499. Novosibirsk, pp 92–140 (in Russian)
Wild GO (1933) Mitteilung über ein anscheinend neues Berylliumsilikat. Zentralbl Mineral Geol Paläont 1933A:38–39
Wood DL, Nassau K (1967) Infrared spectra of foreign molecules in beryl. J Chem Phys 47:2220–2228
Yoshioka Y, Jordan KD (1981) Stabilities and structures of the Li–CO2 and Na–CO2 complexes. Chem Phys Lett 84:370–374
Zeldes H (1963) Paramagnetic species in irradiated KNO3. Paramagnetic resonance, Proceedings of the 1st international conference Jerusalem 1962. Academic Press, New York, pp 764–784
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00269-010-0364-5
Rights and permissions
About this article
Cite this article
Andersson, L.O. EPR investigation of NO2 and CO2 − and other radicals in beryl. Phys Chem Minerals 37, 435–451 (2010). https://doi.org/10.1007/s00269-009-0345-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-009-0345-8