Abstract
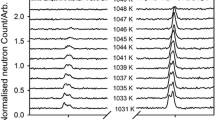
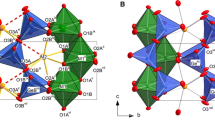
The cell dimensions and crystal structures of the fluoroperovskite NaMgF3 (neighborite), synthesized by solid state methods, have been determined by powder neutron diffraction and Rietveld refinement over the temperature range 300–3.6 K using Pt metal as an internal standard for calibration of the neutron wavelength. These data show that Pbnm NaMgF3 does not undergo any phase transitions to structures of lower symmetry with decreasing temperature. The cell dimensions and atomic coordinates together with polyhedron volumes and distortion indices are given for Pbnm NaMgF3 at 25 K intervals from 300 to 3.6 K. Decreases in the a and c cell dimensions reach a saturation point at 50 K, whereas the b dimension becomes saturated at 150 K. The distortion of the structure of Pbnm NaMgF3 from the aristotype cubic \( Pm\ifmmode\expandafter\bar\else\expandafter\=\fi{3}m \) structure is described in terms of the tilting of the MgF6 octahedra according to the tilt scheme a − a − c +. With decreasing temperature the antiphase tilt (a −) increases from 14.24° to 15.39°, whereas the in-phase tilt (c +) remains effectively constant at ∼10.7°. Changes in the tilt angles are insufficient to cause changes in the coordination sphere of Na that might induce a low temperature phase transition. The structure of Pbnm NaMgF3 is also described in terms of normal mode analysis and displacements of the condensed normal modes are compared with those of Pbnm KCaF3.






Similar content being viewed by others
References
Andrault D, Poirier JP (2002) Evolution of distortion of perovskites under pressure. An EXAFS study of BaZrO3, SrZrO3, and CaGeO3. Phys Chem Mineral 18:91–105
Avdeev M, Caspi EN, Yakovlev S (2007) On the polyhedral volume ratios VA/VB in perovskites ABX 3. Acta Cryst B63:363–372
Balić-Zunić T, Vickovic I (1996) IVTON—a program for the calculation of geometrical aspects of crystal structures. J Appl Crystallogr 29:305–306
Berastegui P, Hull S, Eriksson SG (2001) A low temperature structural phase transition in CsPbF3. J Phys Condens Matter 13:5077–5088
Carpenter MA, Howard CJ, Kennedy BJ, Knight KS (2005) Strain mechanism for order parameter coupling through successive phase transitions in PrAlO3. Phys Rev B72:024118
Chakhmouradian AR, Ross K, Mitchell RH, Swainson I (2001) The crystal chemistry of synthetic potassium-bearing neighborite, (Na1-x K x )MgF3. Phys Chem Mineral 28:277–284
Chen J, Liu H, Martin CD, Parise JB, Weidner DJ (2005) Crystal chemistry of NaMgF3 perovskite at high pressure and temperature. Am Mineral 90:1534–1539
Darlington CNW (2002a) Normal mode analysis of the structures of perovskites with tilted octahedra. Acta Cryst A58:66–71
Darlington CNW (2002b) Normal mode analysis of the structures of perovskites with tilted octahedra. Acta Cryst A58:299–300 (Erratum)
Glazer AM (1972) The classification of tilted octahedra in perosvkites. Acta Cryst B28:3384–3392
Goldschmidt HJ, Land T (1947) An X-ray investigation of the embrittlement of platinum and palladium–rhodium wires. J Iron Steel Inst 155:221–226
Howard CJ, Stokes HT (1998) Group theoretical analysis of octahedral tilting in perovskites. Acta Cryst B54:782–789
Howard CJ, Stokes HT (2005) Structures and phase transitions in perovskites—a group theoretical approach. Acta Cryst A61:93–111
Kern AA, Coelho AA (1998) TOPAS version 2.1: general profile and structure analysis software for powder diffraction data. Bruker AXS Karlsruhe, p 79
Knight KS, Darlington CNW, Wood IG (2005) The crystal structure of KCaF3 at 4.2 and 300 K: a re-evaluation using high resolution powder neutron diffraction. Powder Diffr 20:7–13
Knight KS, Bonanos N, Darlington CNW (2007) Structural and thermoelastic study of the protonic conductor Sr Ce0.95Y0.05Oξ (ξ ∼ 3). J. Solid State Chem (submitted)
Larsen AC, Von Dreele RB (2004) General structure analysis system (GSAS). Los Alamos National Laboratory Report
Martin CD, Chaudhuri S, Grey CP, Parise JB (2005) Effect of A-site cation radius on the ordering of BX6 octahedra in (K,Na)MgF6 perovskite. Am Mineral 90:1522–1533
Mitchell RH (2002) Perovskites: modern and ancient. Almaz Press, Thunder Bay, p 318. (http://www.almazpress.com)
Mitchell RH, Swainson IP (2004) Crystal structure of the fluoroperovskite KMgF3 at 20 K Steacie Institute for Molecular Sciences. Neutron Program for Materials Research, Annual Report, pp 40–41
Mitchell RH, Cranswick LMD, Swainson I (2006) Neutron diffraction determination of the cell dimensions and thermal expansion ofthe fluoroperovskite KMgF3 from 293 to 3.6 K. Phys Chem Minerals 33:587–594
Muradyan LA, Zavodnik VE, Makarova EP, Alexsandrov KS, Simonov VI (1984) Thermal vibrations of atoms in the structure of KMgF3 at 293 and 123 K. Kristallografiya 29:392–394
Pischedda V, Ferraris G, Raade G (2005) Single crystal X-ray diffraction study on neighborite (NaMgF3) from Gjerdingselva, Norawy. Neus Jahrbuch Mineral Abhand 182:23–29
Rönnenbrö E, Noreus D, Kadir K, Reiser A, Bogdanovic B (2000) Invesitigation of the perovskite related structures of NaMgH3, NaMgF3, and Na3AlH6. J Alloys Compd 299:101–106
Shannon RD (1967) Revised effective ionic radii and systematic studies of interatomic distances in halides and chlacogenides. Acta Cryst A32:751–767
Thomas NW (1998) A new global parametrization of perovskite structures. Acta Cryst B54:855–599
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J App Cryst 34:210–213
Touloukian YS, Kirby RK, Taylor RE, Desai PD (1975) Thermophysical properties of matter. Thermal expansion: metallic elements and alloys, vol 12. Plenum Press, New York, pp 254–259
Wood IG, Knight KS, Price GD, Stuart JA (2002) Thermal expansion and atomic displacement parameters of cubic KMgF3 determined by high resolution neutron powder diffraction. J Appl Crystallogr 35:291–29
Yoshiasa A, Sakamoto D, Okudera H, Ohkawa H, Ota K (2003) Phase relations of Na1-x K x MgF3 (0 ≤ x ≤ 1) perovskite-type solid solutions. Mater Res Bull 38:421–427
Zhao Y (1998) Crystal chemistry and phase transitions of perovskites in P-T-X space: data for (Na1-x K x ) MgF3 perovskites. J Solid State Chem 141:121–132
Zhao Y, Weidner DJ, Parise JB, Cox DE (1993a) Thermal expansion and structural distortion of perovskite—data for NaMgF3. Part I. Phys Earth Planet. Interiors 76:1–16
Zhao Y, Weidner DJ, Parise JB, Cox DE (1993b) Critical phenomens and phase transition of perovskite—data for NaMgF3 perovskite. part II. Phys. Earth Planet. Interiors 76:17–34
Zhao Y, Parise JB, Wang Y, Kusaba K, Vaughan MT, Weidner DJ, Kikegawa T, Chen J, Shimomura O (1994) High pressure crystal chemistry of neighborite NaMgF3 perovskite: an angle-dispersive diffraction study using monochromatic synchrotron X-radiation. Am Mineral 79:615–621
Acknowledgments
This work is supported by the Natural Sciences and Engineering Research Council of Canada and by Lakehead University. Raymond Sammon and Travis Dodd are thanked for the setup of the closed cycle refrigerator at the Chalk River C2 spectrometer. Kevin Knight (ISIS Facility, Rutherford Appleton Laboratory) is especially thanked for assistance with and discussion of the normal mode analysis of perovskite structures. Two anonymous reviewers are thanked for comments on the original draft of this paper. Catherine McCammon is thanked for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitchell, R.H., Alexander, M., Cranswick, L.M.D. et al. A powder neutron diffraction study of the crystal structure of the fluoroperovskite NaMgF3 (neighborite) from 300 to 3.6 K. Phys Chem Minerals 34, 705–712 (2007). https://doi.org/10.1007/s00269-007-0188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-007-0188-0