Abstract
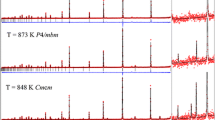
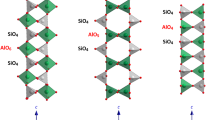
The nature of the apparently continuous structural phase transition at 1,049 K in the perovskite-structured, MgSiO3 isomorph, neighborite (NaMgF3), from the orthorhombic (Pbnm) hettotype phase to the cubic (\( Pm\overline{3} m \)) aristotype structure, has been re-investigated using high-resolution, time-of-flight neutron powder diffraction. Using data collected at 1 K intervals close to the nominal phase transition temperature, the temperature dependence of the intensities of superlattice reflections at the M point \( \left( {\frac{2\pi }{a}\left[ {\frac{1}{2},\frac{1}{2},0} \right]} \right) \) and the R point \( \left( {\frac{2\pi }{a}\left[ {\frac{1}{2},\frac{1}{2},\frac{1}{2}} \right]} \right) \) of the pseudocubic Brillouin zone indicate the existence of a new intermediate tetragonal phase in space group P4/mbm, with a narrow phase field extending from ~1,046.5 to ~1,048.5 K, at ambient pressure. Group theoretical analysis shows that the structural transitions identified in this study, Pbnm–P4/mbm, and P4/mbm–\( Pm\overline{3} m \), are permitted to be second order. The observation of the tetragonal phase resolves the longstanding issue of why the high-temperature phase transition, previously identified as Pbnm–\( Pm\overline{3} m \), and which would be expected to be first order under Landau theory, is in fact found to be continuous. Analysis of the pseudocubic shear strain shows it to vary with a critical exponent of 0.5 implying that the phase transition from Pbnm to P4/mbm is tricritical in character. The large librational modes that exist in the MgF6 octahedron at high temperature, and the use of Gaussian probability density functions to describe atomic displacements, result in apparent bond shortening in the Mg–F distances, making mode amplitude determination an unreliable method for determination of the critical exponent from internal coordinates. Crystal structures are reported for the three phases of NaMgF3 at 1,033 K (Pbnm), 1,047 K (P4/mbm) and 1,049 K (\( Pm\overline{3} m \)).



Similar content being viewed by others
References
Adams DJ, Oganov AR (2006) Ab initio molecular dynamics study of CaSiO3 perovskite at P-T conditions of Earth’s lower mantle. Phys Rev B 73:184106
Carpenter MA (2007) Elastic anomalies accompanying phase transitions in (Ca, Sr)TiO3 perovskites: Part I. Landau theory and a calibration for SrTiO3. Am Mineral 92:309–327
Carpenter MA, Salje EKH, Graeme-Barber A (1998) Spontaneous strain as a determinant of thermodynamic properties for phase transitions in minerals. Eur J Mineral 10:619–691
Chao ECT, Evans HT, Skinner BJ, Milton C (1961) Neighborite, NaMgF3, a new mineral from the Green River Formation, South Ouray, Utah. Am Mineral 46:379–393
Chen J, Liu H, Martin CD, Parise JB, Weidner DJ (2005) Crystal chemistry of NaMgF3 perovskite at high pressure and temperature. Am Mineral 90:1534–1539
Cochran W, Zia A (1968) Structure and dynamics of perovskite-type crystals. Phys Status Solidi 25:273–283
Cowley RA (1964) Lattice dynamics and phase transitions of strontium titanate. Phys Rev 134:A981–A997
Dobson DP, Hunt SA, Lindsay-Scott A, Wood IG (2011) Towards better analogues for MgSiO3 post-perovskite: NaCoF3 and NaNiF3, two new recoverable fluoride post- perovskites. Phys Earth Planet Inter 189:171–175
Dobson DP, Miyajima N, Nestola F, Alvaro M, Casati N, Liebske C, Wood IG, Walker AM (2013) Strong inheritance of texture between perovskite and post-perovskite in the D′′ layer. Nat Geosci 6:575–578
Glazer AM (1972) The classification of tilted octahedra in perovskites. Acta Crystallogr B 28:3384–3392
Glazer AM (1975) Simple ways of determining perovskite structures. Acta Crystallogr A 31:756–762
Grocholski B, Shim S-H, Prakapenka VB (2010) Stability of the MgSiO3 analog NaMgF3 and its implication for mantle structure in super-Earths. Geophys Res Lett 37:L14204
Howard CJ, Stokes HT (1998) Group-theoretical analysis of octahedral tilting in perovskites. Acta Crystallogr B 54:782–789
Hustoft J, Catalli K, Shim S-H, Kubo A, Prakapenka VB, Kunz M (2008) Equation of state of NaMgF3 postperovskite: implications for the seismic velocity changes in the D′′ region. Geophys Res Lett 35:L10309
Ibberson RM, David WIF, Knight KS (1992) Report RAL-92-031. Rutherford Appleton Laboratory, Didcot
Knight KS (2009) Parameterization of the crystal structures of centrosymmetric zone-boundary-tilted perovskites: an analysis in terms of symmetry-adapted basis-vectors of the cubic aristotype phase. Can Mineral 47:381–400
Knight KS (2011) Centrosymmetric perovskite crystal structures with space group Pbnm: crystallographic parameterization of KCaF3 between 100 and 400 K in terms of the amplitudes of symmetry-adapted basis-vectors of the cubic aristotype phase. Can Mineral 49:793–808
Knight KS (2014) A high resolution neutron diffraction study of the crystal structure of neighborite (NaMgF3) between 9 and 440 K. Am Mineral 99:824–838
Knight KS, Darlington CNW, Wood IG (2004) The crystal structure of KCaF3 at 4.2 and 300 K: a re-evaluation using high-resolution powder neutron diffraction. Powder Diffr 20:7–13
Komabayashi T, Hirose K, Sata N, Ohishi Y, Dubrovinsky LS (2007) Phase transition in CaSiO3 perovskite. Earth Planet Sci Lett 260:564–569
Larson AC, Von Dreele RB (1986) GSAS, general structure analysis system. Los Alamos National Laboratory Report No. LAUR 86–748
Li L, Weidner DJ (2012) Anelasticity and transient creep in NaMgF3 perovskite at high pressure. Phys Earth Planet Inter 194–195:98–106
Li L, Weidner DJ, Brodholt J, Alfè D, Price GD, Caracas R, Wentzcovitch R (2006) Phase stability of CaSiO3 perovskite at high pressure and temperature: insights from ab initio molecular dynamics. Phys Earth Planet Inter 155:260–268
Liu H-Z, Chen J, Hu J, Martin CD, Weidner DJ, Häusermann D, Mao H-K (2005) Octahedral tilting evolution and phase transition in orthorhombic NaMgF3 perovskite under pressure. Geophys Res Lett 32:L04304
Martin CD, Chaudhuri S, Grey CP, Parise JB (2005) Effect of A-site cation radius on ordering of BX6 octahedra in (K, Na)MgF3 perovskite. Am Mineral 90:1522–1533
Martin CD, Crichton WA, Liu HZ, Prakapenka V, Chen JH, Parise JB (2006a) Phase transitions and compressibility of NaMgF3 (Neighborite) in perovskite and post-perovskite-related structures. Geophys Res Lett 33:L11305
Martin CD, Crichton WA, Liu H, Prakapenka V, Chen J, Parise JB (2006b) Rietveld structure refinement of perovskite and post-perovskite phases of NaMgF3 (Neighborite) at high pressures. Am Mineral 91:1703–1706
Martin CD, Chupas PJ, Chapman KW, Parise JB (2007) Local versus average structure: a study of neighborite (NaMgF3) utilizing the pair distribution function method for structure determination. J Appl Crystallogr 40:441–448
McKnight REA, Howard CJ, Carpenter MA (2009) Elastic anomalies with transformation sequencies in perovskites: I. Strontium zirconate, SrZrO3. J Phys-Condens Mat 21:015901
Mitchell RH, Alexander M, Cranswick LMD, Swainson IP (2007) A powder neutron diffraction study of the fluoroperovskite NaMgF3 (neighborite) from 300 to 3.6 K. Phys Chem Minerals 34:507–712
Murakami M, Hirose K, Kawamora K, Sata N, Ohishi Y (2004) Post perovskite phase transition in MgSiO3. Science 304:855–858
Noguchi N, Komabayashi T, Hirose K, Ohishi Y (2013) High-temperature compression experiments of CaSiO3 perovskite to lowermost mantle conditions and its thermal equation of state. Phys Chem Minerals 40:81–91
Oganov AR, Ono S (2004) Theoretical and experimental evidence for a post-perovskite phase of MgSiO3 in Earth’s D′′ layer. Nature 430:445–448
Perez-Mato JM, Orobengoa D, Aroyo MI (2010) Mode crystallography of distorted structures. Acta Crystallogr A 66:558–590
Poirier JP, Peyronneau J, Gesland JY, Brebec G (1983) Viscosity and conductivity of the lower mantle: an experimental study on a MgSiO3 perovskite analogue, KZnF3. Phys Earth Planet Inter 32:273–287
Stokes HT, Hatch DM (1988) Isotropy subgroups of the 230 crystallographic space groups. World Scientific Press, Singapore 603 pp
Street JN, Wood IG, Knight KS, Price GD (1997) The influence of thermal vibrations on the average structure of cubic NaMgF3 perovskite: a combined molecular dynamics and neutron diffraction study. J Phys: Condens Matter 9:L647–L655
Tolédano J-C, Tolédano P (1987) The Landau theory of phase transitions. World Scientific Press, Singapore 451 p
Torpor L, Navrotsky A, Zhao Y, Weidner DJ (1997) Thermochemistry of fluoride perovskites: heat capacity, enthalpy of formation, and phase transition of NaMgF3. J Solid State Chem 132:131–138
Uchida T, Wang Y, Nishiyama N, Funakoshi K, Kaneko H, Nozawa A, Von Dreele RB, Rivers ML, Sutton SR, Yamada A, Kunimoto T, Irifune T, Inoue T, Li B (2009) Non-cubic symmetry of CaSiO3 perovskite up to 18 GPa and 1600 K. Phys Earth Planet Inter 282:268–274
Umemoto K, Wentzcovitch RM (2006) Potential ultrahigh pressure polymorphs of ABX3 compounds. Phys Rev B 74:224105
Umemoto K, Wentzcovitch RM, Weidner DJ, Parise JB (2006) NaMgF3: a low pressure analog of MgSiO3. Geophys Res Lett 33:L15304
Watson GW, Wall A, Parker SC (1995) A molecular dynamics simulation of the effect of high pressure on fast-ion conduction in a MgSiO3-perovskite analogue; KCaF3. Phys Earth Planet Inter 89:137–144
Zhao Y, Weidner DJ, Parise JB, Cox DE (1993a) Thermal expansion and structural distortion of perovskite: data for NaMgF3 perovskite. Part I. Phys Earth Planet Inter 76:1–16
Zhao Y, Weidner DJ, Parise JB, Cox DE (1993b) Critical phenomena and phase transition of perovskite—data for NaMgF3 perovskite. Part II. Phys Earth Planet Inter 76:17–34
Acknowledgments
We are grateful for the careful reviews of this article by Prof. M. A. Carpenter and an anonymous reviewer. This work was supported by the Natural Environment Research Council (ref. NE/J009520/1). IGW thanks J. P. Brodholt and D. P. Dobson for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knight, K.S., Price, G.D., Stuart, J.A. et al. High-temperature structural phase transitions in neighborite: a high-resolution neutron powder diffraction investigation. Phys Chem Minerals 42, 45–52 (2015). https://doi.org/10.1007/s00269-014-0698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-014-0698-5