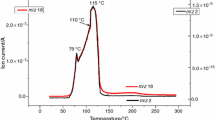
Abstract
A series of natural omphacites from a wide range of P, T occurrences were investigated by electron microprobe (EMP), infrared (IR)-, Mössbauer (MS)- and optical spectroscopy in the UV/VIS spectral range (UV/VIS), secondary ion mass spectrometry (SIMS) and single crystal structure refinement by X-ray diffraction (XRD) to study the influence of hydrogen loss on valence state and site occupancies of iron. In accordance with literature data we found Fe2+ at M1 as well as at M2, and in a first approach assigned Fe3+ to M1, as indicated by MS and XRD results. Hydrogen content of three of our omphacite samples were measured by SIMS. In combination with IR spectroscopy we determined an absorption coefficient: ε i,tot = 65,000 ± 3,000 lmolH2O −1 cm−2. Using this new ε i,tot value, we obtained water concentrations ranging from 60 to 700 ppm H2O (by weight). Hydrogen loss was simulated by stepwise heating the most water rich samples in air up to 800°C. After heat treatment the samples were analyzed again by IR, MS, UV/VIS, and XRD. Depending on the type of the OH defect, the grade of dehydration with increasing temperature is significantly different. In samples relatively poor in Fe3+ (<0.1 Fe3+ pfu), hydrogen associated with vacancies at M2 (OH bands around 3,450 cm−1) starts to leave the structure at about 550°C and is completely gone at 780°C. Hydrogen associated with Al3+ at the tetrahedral site (OH bands around 3,525 cm−1, Koch-Müller et al., Am Mineral, 89:921–931, 2004) remains completely unaffected by heat treatment up to 700°C. But all hydrogen vanished at about 775°C. However, this is different for a more Fe3+-rich sample (0.2 Fe3+ pfu). Its IR spectrum is characterized by a very intense OH band at 3,515 cm−1 plus shoulder at 3,450 cm−1. We assign this intense high-energy band to vibrations of an OH dipole associated with Fe3+ at M1 and a vacancy either at M1 or M2. OH release during heating is positively correlated with decrease in Fe2+ and combined with increase in Fe3+. That dehydration is correlated with oxidation of Fe2+ is indirectly confirmed by annealing of one sample in a gas mixing furnace at 700°C under reducing conditions keeping almost constant OH− content and giving no indication of Fe2+-oxidation. Obtained data indicate that in samples with a relatively high concentration of Fe2+ at M2 and low-water concentrations, i.e., at a ratio of Fe2+ M2/H > 10 dehydration occurs by iron oxidation of Fe2+ exclusively at the M2 site following the reaction: \( {\left[ {{\text{Fe}}^{{{\text{2 + [ M2]}}}}{\text{OH}}^{ - } } \right]} = {\left[ {{\text{Fe}}^{{{\text{3 + [ M2]}}}} {\text{O}}^{{{\text{2}} - }} } \right]} + {\text{1/2}}\;{\text{H}}_{{\text{2}}} \uparrow . \) In samples having relatively low concentration of Fe2+ at M2 but high-water concentrations, i.e., ratio of Fe2+ M2/H < 5.0 dehydration occurs through oxidation of Fe2+ at M1.









Similar content being viewed by others
References
Abs-Wurmbach I, Amthauer G (1988) Crystal chemistry of iron in natural and in synthetic braunites Mn2+(Mn3+, Fe3+)6O8/SiO4. Z Krist 1984:13–30
Bell DR, Ihinger PH, Rossman GR (1995) Quantitative analysis of trace OH in garnet and pyroxenes. Am Miner 80:465–474
Blanchard M, Ingrin J (2004) Hydrogen diffusion in Dora Maira pyrope. Phys Chem Miner 31:593–605
Burns RG (1993) Mineralogical applications of crystal field theory, 2nd edn. Cambridge University Press, Cambridge
Carpenter MA (1983) Microstructures in sodic pyroxenes; implications and applications. In: Proceedings of the meeting on application of mineral synthesis to high-pressure petrology; the real and average structures of synthetic and natural pyroxenes and amphiboles. Istituto di Mineralogia dell’Universita di Roma, Rome, Italy, pp 271–301
Dobretsov NL, Sobolev NV, Schatskiy WS et al (1989) Eclogite and glaucophane schist of fold mountains. Nauka, Novosibirsk, 236p
Dyar MD, McGuire AV, Ziegler RD (1989) Redox equilibria and crystal chemistry of coexisting minerals from spinel lherzolite mantle xenoliths. Am Miner 74:969–980
Franz G, Thomas S, Smith DC (1986) High-pressure phengite decomposition in the Weissenstein eclogite, Münchberger Gneiss Massif, Germany. Contrib Mineral Petrol 92:71–85
Hammerschmidt K, Franz G (1992) Retrograde evolution of eclogites; evidences from microstructures and 40Ar/39Ar white mica dates, Muenchberg Massif, northern Bavaria. Contrib Mineral Petrol 111:113–125
Hercule S, Ingrin J (1999) Hydrogen in diopside: diffusion, kinetics of extraction-incorporation, and solubility. Am Miner 84:1577–1587
Ingrin J, Skogby H (2000) Hydrogen in nominally anhydrous upper-mantle minerals: concentration levels and implications. Eur J Miner 12:543–570
Katayama I, Nakashima S, Yurimoto H (2006) Water content in natural eclogite and implication for water transport into the deep upper mantle. Lithos 86:245–259
Koch-Müller M, Matsyuk SS, Rhede D, Wirth R, Khisina N (2006) Hydroxyl in mantle olivine xenocrysts from the Udachnaya kimberlite pipe. Phys Chem Miner 33:276–287
Koch-Müller M, Matsyuk SS, Wirth R (2004) Hydroxyl in omphacites and omphacitic clinopyroxenes of upper mantle to lower crustal origin beneath the Siberian platform. Am Miner 89:921–931
Koch-Müller M, Abs-Wurmbach I, Bubenik W (1999) Intracrystalline fractionation of Fe in synthetic (Fe, Mg, Zn)—bearing staurolite; a Mössbauer spectroscopic study. Phys Chem Miner 26:312–321
Langer K (1988) UV tio NIR spectra of silicate minerals obtained by microscope spectrometry and their use in mineral thermodynamics and kinetics. In: Salje (ed) Physical properties and thermodynamic behaviour of minerals. D. Reidel Publishing Company, Dordrecht, pp 639–685
Li YL, Zheng YF, Fu B (2005) Mössbauer spectroscopy of omphacite and garnet pairs from eclogites; application to geothermobarometry. Am Miner 90:90–100
Libowitzky E, Rossman GR (1997) An IR absorption calibration for water in minerals. Am Miner 82:1111–1115
Maldener J, Hösch A, Langer K, Rauch F (2003) Hydrogen in some natural garnets studied by nuclear reaction analysis and vibrational spectroscopy. Phys Chem Miner 30:337–344
Nagel D (1990) The program “MBF”. Universität Marburg, Germany
Oberti R, Caporuscio FA (1991) Crystal chemistry of clinopyroxenes from mantle eclogites: a study of the key role of the M2 site population by means of crystal- structure refinement. Am Miner 76:1141–1152
Paterson MS (1982) The determination of hydroxyl by infrared absorption in quartz, silicate glasses and similar materials. Bull Minér 105:20–29
Pouchou JL, Pichoir F (1985) “PAP” F(ρZ) procedure for imporved quantitative microanalysis. Microbeam Anal 1985:104–106
Raheim A, Green DH (1974) Experimental determination of the temperature and pressure dependence of the Fe-Mg partition coefficient for coexisting garnet and clinopyroxene. Contrib Miner Petrol 48:179–203
Rhede D, Wiedenbeck M (2006) SIMS Quantification of Very Low H2O Contents. Appl Surf Sci 252:7152–7154
Rossi G, Oberti R, Dal NA, Molin GM, Mellini M (1987) Residual electron density at the M2 site in C2/c clinopyroxenes; relationships with bulk chemistry and sub-solidus evolution. Phys Chem Miner 14:514–520
Sachs L (1972) Statistische auswertungmethoden, 3rd edn error progagation. Springer, Berlin, Heidelberg, New York, p 78
Skogby H, Rossman GR (1989) OH in pyroxene: an experimental study of incorporation mechanisms and stability. Am Miner 74:1059–1069
Stalder R, Skogby H (2007) Dehydration mechanisms in synthetic Fe-bearing enstatite. Eur J Miner 19:201–216
Woods SC, Mackwell S, Dyar D (2000) Hydrogen in diopside: diffusion profiles. Am Miner 85:480–487
Wright SE, Foley JA, Hughes JM (2000) Optimization of site occupancies in minerals using quadratic programming. Am Miner 85:524–531
Yakovlev BG, Matsyuk SS, Wischnewskiy AA, Tschubarov WM, Spetsius ZW (1990) The evolution of mineral equilibria and petrogenesis of the deep mafic ferruginous granulites from Yakutian kimberlite piepes. Mineralogicheskiy J 12:N4:3–15 (in Russian)
Acknowledgments
We thank R. Schulz, O. Appelt, and I. Schäpan for assistance in the different labs. Prof. G. Franz is thanked for providing some of the natural samples and Dr. M. Wiedenbeck for helpful discussions and suggestions concerning the SIMS measurements. The comments and suggestions of R. Stalder and an anonymous reviewer helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch-Müller, M., Abs-Wurmbach, I., Rhede, D. et al. Dehydration experiments on natural omphacites: qualitative and quantitative characterization by various spectroscopic methods. Phys Chem Minerals 34, 663–678 (2007). https://doi.org/10.1007/s00269-007-0181-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-007-0181-7