Abstract
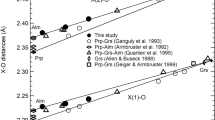
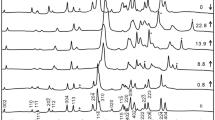
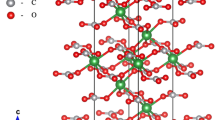
A laser-heated diamond-anvil cell that is capable of operating up to a pressure of 63 GPa, with X-ray diffraction facilities using a synchrotron radiation source at the SPring-8, has been developed to observe the compressibility of a hexagonal aluminous phase, [K0.15Na1.66Ca0.11Mg1.29Fe2+ 0.86Al3.13Ti0.09Si1.98] Σ9.27O12. The hexagonal aluminous phase is a potassium host mineral from the subducted oceanic crust in the Earth's lower mantle. A sample was heated using a YAG laser at each pressure increment to relax the deviatoric stress in the sample. X-ray diffraction measurements were carried out at 300 K using an angle-dispersive technique. Pressure was measured using an internal platinum pressure calibrant. The observed unit-cell volumes were used to obtain a third-order Birch–Murnaghan equation of state: unit-cell volume V o=185.94(±16) Å3, density ρ o=4.145 g/cm3, and bulk modulus K o=198(±3) GPa when the first pressure is derivative of the bulk modulus K ′ o is fixed to 4. The density of hexagonal aluminous phase is lower than that of coexisting Mg-perovskite in the subducted oceanic crust.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 14 December 2001 / Accepted: 26 June 2002
Rights and permissions
About this article
Cite this article
Ono, S., Hirose, K., Isshiki, M. et al. Equation of state of hexagonal aluminous phase in basaltic composition to 63 GPa at 300 K. Phys Chem Min 29, 527–531 (2002). https://doi.org/10.1007/s00269-002-0263-5
Issue Date:
DOI: https://doi.org/10.1007/s00269-002-0263-5