Abstract
Background
Current literature describing the riskiness of operating on actively infected COVID-19 patients far outnumbers that on the risk of operating on recovered patients. The purpose of this study was to analyze a single, tertiary referral center experience regarding postoperative complications and readmissions in COVID-19-recovered patients versus COVID-19-naïve (never previously infected) patients undergoing elective and emergency surgery across all surgical subspecialties.
Methods
All PCR positive COVID-19 patients that underwent a surgical procedure between February 1, 2020, and November 1, 2020, were included in the COVID-positive cohort. These patients were then matched to COVID-naïve controls that underwent similar procedures within the same time frame. Primary outcomes included 30-day postoperative complications as well as 90-day readmissions. Multivariable analyses were also performed.
Results
112 COVID-positive patients met inclusion criteria and were all matched to COVID-naïve controls. 76 patients (68%) underwent surgery > 30 days from their COVID diagnosis. COVID-positive patients were at significantly higher risk of 30-day complications compared to the COVID-naïve cohort (22% versus 8%, respectively; p < 0.01). Multivariable analyses found ambulatory/asymptomatic infections, undergoing surgery between 30 and 120 days from diagnosis, initial presentation to the emergency department and elevated ASA scores to be significantly associated with 30-day complications. No differences were found for 90-day readmissions.
Conclusion
Patients with previous COVID-19 infections carry a higher perioperative risk profile for 30-day complications compared to COVID-naïve counterparts in unvaccinated populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SARS-CoV-2 (COVID-19) has infected over 400 million people worldwide, with a death toll surging over 5 million [1]. During the “first wave” of early spring 2020 in the USA, most facilities were forced to stratify risk and discontinue elective procedures in order to protect patients, surgeons and operating room staff as well as siphon both equipment and manpower to where it was needed more [2,3,4].
Despite this, emergent surgery continued, allowing for a glimpse into the perioperative influence of the virus. It has since been well described that operating on patients with active or incubating COVID-19 infections results in increased perioperative risk including increased pulmonary complications, thrombotic complications, unplanned intensive care unit admissions and 30-day mortality [5,6,7,8,9,10]. A May 2020 study demonstrated pulmonary complications occurred in over half of the cohort [5] requiring surgeons to think more critically before performing non-urgent cases on actively infected patients.
With continued outbreaks of COVID-19 and the advent of vaccinations, society continues to navigate reopening safely. Many hospital systems across the globe have resumed elective surgery under the policy of the patient having a negative preoperative COVID-19 PCR test, hoping to mitigate the aforementioned heightened risk in active infections [11]. However, with a significant portion of the world’s population having already contracted the virus, the majority of which are recovered [1], the non-infected COVID-19 “survivor” is a patient that will more commonly begin to be encountered in clinics. Currently, the breadth of data on active infections far exceeds that on previous infections resulting in a need for clarity on how to counsel patients as to when it may be safe to perform elective surgery as well as how their postoperative course could be affected by previous infection. Therefore, the purpose of this study is to analyze a single, tertiary referral center experience regarding postoperative complications and readmissions in COVID-19 positive and recovered patients versus COVID-naïve (never previously infected) patients undergoing elective and emergency surgery across all surgical subspecialties.
Methods
Data source
This is a single center, retrospective cohort study from a high-volume tertiary referral center in the USA. After approval by the Institutional Review Board, the electronic medical record (EMR) was queried for all patients over 18 years of age that had a COVID-19 diagnosis and had a surgical procedure of any kind between the dates of February 1, 2020, and November 1, 2020. We chose this study period to evaluate the time period prior to the availability of vaccination to isolate the effects of prior COVID-19 infection in unvaccinated patients. A COVID-19 diagnosis was defined as a positive test result by polymerase chain reaction (PCR) testing. This was identified via ICD 10 code U07.1. Individual chart review allowed for subclassification of infection severity based on admission status and symptomatology. Our geographic region consists of two major health systems with an integrated inpatient and outpatient EMR that allows for comprehensive review of laboratory data, inpatient admissions, and outpatient visits for the region. Ambulatory infections were defined as those that did not require a hospital admission. Recovered status was used to describe those 30 days from a positive test. A surgical procedure was defined as an anesthesia event involving general anesthesia or monitored anesthesia care (MAC). Both inpatient and outpatient surgical procedures were included. Patients were required to have a COVID-19 diagnosis prior to surgery regardless of time elapsed. Exclusion criteria included children (< 18 years old) and those who chronologically underwent surgery prior to COVID-19 diagnosis.
Matching
Once the primary cohort had been assembled via the above noted procedures, the electronic medical record was re-queried to obtain matched COVID-naïve controls. This was performed by searching for the CPT code coinciding to each surgical procedure performed during the same time period in patients without a positive COVID-19 test prior to their procedure. The query was then further narrowed by sex. Matched, deidentified, patients were then selected, first prioritizing age and surgeon subspecialty and additionally race/ethnicity and surgeon when able. Additional exclusions from the COVID-positive cohort were then made at this time if no COVID-naïve match existed. Similar variables were then recorded excluding those related to COVID-19 diagnosis.
Outcomes
Primary postoperative outcomes included 30-day complications and 90-day hospital readmissions. Complications included non-infectious pulmonary complications (such as hypoxia and prolonged intubation), pneumonia, cardiac complications (such as myocardial infarction and congestive heart failure), genitourinary (such as urinary tract infection and kidney stones), cerebrovascular accident, sepsis and emergency department visits without definitive diagnoses.
Key exposure variable and covariates
The key exposure variable was a binary indicator of whether a patient had a COVID-19 diagnosis or not defined using the methodology described above. Patient demographics were recorded including sex, age, race, ethnicity, body mass index (BMI), American Society of Anesthesiologists (ASA) score, Charlson Comorbidity Index (CCI), and smoking status. Procedure-specific variables included procedure performed, surgeon subspecialty, and location of preoperative presentation (clinic versus emergency department). The latter was used as a surrogate to define elective versus emergent procedures.
Statistical analysis
For each patient and procedure-specific variable, COVID-positive patients were compared to COVID-naïve patients using the Kruskal–Wallis, and Chi-squared tests for continuous and categorical variables, respectively. We estimated multivariable logistic models to infer associations of the outcomes with the key exposure variable and the covariates. We report the odds ratios for the logistic regression models. All analyses were performed using Stata MP 16.1 (StataCorp LLC, College Station, TX). p values < 0.05 were considered statistically significant. Analyses were performed for all patients as well as those > 30 days from COVID diagnosis and their matched counterparts to isolate those further from active infection.
Sensitivity analysis
We re-estimated separate multivariable models by replacing the binary indicator for COVID-19 diagnosis with (i) the severity of COVID-19 infection, and (ii) days between positive PCR test and surgery. These multivariable models were otherwise identical to those described in the main ‘Statistical Analysis’ section.
Results
Study cohort
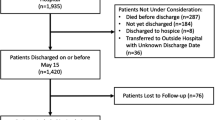
On initial query, 155 patients were identified that had been diagnosed with COVID-19 and undergone a procedure during the study period. 20 patients were excluded due to lack of general anesthesia or MAC during their procedures. An additional 23 patients were subsequently excluded after no appropriate COVID-naïve counterpart was able to be identified. The final cohorts consisted of 112 COVID-positive patients successfully matched 1:1 to COVID-naïve controls (Fig. 1).
Unadjusted analysis
Both cohorts included 67 women and 45 men (Table 1). Mean age for the COVID-positive cohort was 55.9 years compared to 56.1 years for the COVID-naive. Both cohorts were predominately White and of non-Hispanic ethnicity. There were no significant differences in the distribution of BMI, CCI, and smoking status between the two groups.
Orthopedic surgery and general surgery were the most common subspecialties (Table 2). In both cohorts, more patients presented in the ambulatory setting and deemed elective (57% and 64% in the COVID-positive and COVID-naïve cohorts, respectively) compared to those undergoing urgent/emergent procedures through the emergency department (43% and 36% in the COVID-positive and COVID-naïve cohorts, respectively). Within the COVID-positive cohort, 76% of patients were ambulatory during their infection with 57% being asymptomatic. 24% of the cohort required hospitalization with 8% requiring ICU level of care. Mean time between COVID-19 diagnosis and surgery was 81.8 days (standard deviation [SD]: 69.1 days). 76 patients (68%) underwent surgery more than 30 days from diagnosis. 20 patients (17.9%) underwent surgery within 10 days of diagnosis.
COVID-positive patients had a higher 30-day complication rate (22% versus 8%, p = 0.003) than the COVID-naïve (Table 3). After excluding those < / = 30 days from diagnosis, similar trends were noted for 30-day complications (18.5% for COVID-positive versus 7.4% for COVID-naïve, p = 0.06) although this was not statistically significant. Pulmonary complications occurred most commonly (Table 4). There were no statistically significant differences in readmission rate between COVID-positive and COVID-naïve patients, both in the overall cohort and in patients > 30 days from diagnosis (Table 3).
Multivariable analysis
On multivariable analysis (Table 5), prior COVID-19 infection was associated with 30-day complications for both all patients (odds ratio [OR] 3.10, 95% Confidence Interval [CI]: 1.18–8.12 p = 0.02) as well as those > 30 days from infection (OR 4.71 (1.27–17.49), p = 0.02). The odds of 30-day complications were also higher for patients with higher ASA score (OR 5.43 (2.33–12.67) for all patients, p = < 0.01; OR 7.06 (2.18–22.84) for those > 30 days from diagnosis, p = < 0.01); and for patients presenting to the emergency department compared to the ambulatory setting (OR 4.65 (1.64–13.17) for all patients, p = < 0.01; OR 8.8 (2.27–34.15) for those > 30 days from diagnosis, p = < 0.01).
Prior COVID-19 infection was not a risk factor for 90-day readmissions both in the overall cohort and in patients > 30 days from their diagnosis (Table 6). The odds of 90-day readmission were higher for patients with higher ASA score (OR 3.67 (1.58–8.56) for all patients, p = < 0.01; OR 2.99 (1.12–7.98) for those > 30 days from diagnosis, p = 0.03) (Table 5).
For the sensitivity analyses, patients with an ambulatory, asymptomatic COVID-19 infection demonstrated higher 30-day complications relative to COVID-naïve patients (OR 3.44 (1.11–10.7) for all patients, p = 0.03; OR 7.06 (1.31–38.14) for those > 30 days from diagnosis, p = 0.02) (Appendix 1). Additionally, patients whose COVID-19 infection resulted in a hospitalization/ICU care and were more than 30 days from diagnosis had increased complications after surgery relative to COVID-naïve patients (OR 5.74 (1.07–30.65); p = 0.04) (Appendix 1). Those who underwent surgery between 30 and 120 days from diagnosis were also at heightened risk of 30-day complication (OR 4.62 (1.28–16.69) p = 0.02 for all patients; OR 5.12 (1.18–22.26) p = 0.03 for those > 30 days from diagnosis) (Appendix 2).
The inferences from the sensitivity analysis for the 90-day readmission outcome were consistent with those from the main analysis.
Discussion
When compared to a COVID-naive, matched cohort, patients with history of COVID-19 infection carried a heightened perioperative risk profile with regard to 30-day complications. These results held on sensitivity analysis, also detailing that those undergoing surgery between 30 and 120 days from diagnosis may be at particularly high risk. While this information is of obvious utility to surgeons, anesthesiologists and patients when discussing perioperative risk, it is also relevant to payers and hospital systems as hospital resources and bed availability remain a major determinant in permitting elective surgeries throughout many parts of the United States.
Early perioperative literature focused mainly on the patient undergoing surgery with an active COVID-19 infection and quickly illustrated this to be a risky endeavor. Across nearly all surgical specialties, COVID-positive patients were found to have heightened risk of postoperative mortality, cardio-pulmonary complications, kidney injury, and thromboembolic disease. [5,6,7,8,9,10] Larger scale studies determined mortality rate to be as high as upwards of 20% with complication rate as high as 50% [5]. Many patients also required ICU level of care and experienced a prolonged length of stay compared to healthy counterparts [10]. Similar to this prior literature, we found an overall higher complication rate among patients undergoing surgery with prior COVID-19 infection relative to COVID-naïve patients.
Many patients with COVID-19 have residual symptoms after their infection resolves. Carfi et al. [12] quote that even 60 days from diagnosis as few as 12% of patients are completely symptom-free with 32% of patients experiencing 1 or 2 symptoms and 55% of patients experiencing 3 or more symptoms. While many patients experienced primarily pulmonary symptoms, the acute and chronic sequelae of a COVID-19 infection are multi-system in nature with common involvement of cardiac and renal systems [12, 13]. This is paramount to consider in the setting of added physiologic stress from both an anesthetic and surgical procedure.
Furthermore, our study suggests that presence or persistence of symptoms may not fully describe the physiologic burden of an active or previous infection. In both the overall cohort and those > 30 days from diagnosis, multivariate analysis revealed ambulatory/asymptomatic infections to be significantly associated with 30-day complications. These types of infections should therefore not be undervalued when calculating perioperative risk. Multivariable analysis also revealed those presenting to the ED had significantly higher OR, suggestive that non-elective procedures may be at higher risk of complication. This highlights the value of preoperative medical optimization in these patients, which may have been limited in more urgent or traumatic situations compared to the elective setting.
To date, there has been mixed evidence on the persistence of perioperative risk in recovered patients. A 2021 study in anesthesia favors waiting 7 weeks prior to consideration of elective surgery in a COVID-recovered patient [14], indicating that risk of perioperative mortality and pulmonary complication does not match that of healthy patients until this benchmark [15]. Interestingly, they note that the highest rate of pulmonary complication was observed in those diagnosed between 3 and 4 weeks prior to surgery opposed to 1–2 weeks [15]. A similar trend was noted in our analysis as the OR was highest in the 30–120 day cohort. It is unclear what would account for this phenomenon from a physiologic standpoint. We postulate that physicians may have previously underestimated the more chronic impact of COVID infections beyond the contagious window suggested by quarantine recommendations.
Lal et al. [16] support these data reporting significantly higher rates of postoperative pneumonia, mechanical ventilation, acute respiratory distress syndrome (ARDS), septic shock and ischemic stroke. They do not provide an overall 30-day complication rate, though the highest reported incidence was postoperative pneumonia at 20.6%, which is comparable to our overall complication rate. These findings are refuted by that of Baiocchi et al. [17] who, despite a 30-day complication rate of 15%, found no significant differences in complications and mortality in a surgical oncology demographic. Despite differing conclusions, both of these studies focus primarily on patients more recently infected with COVID-19, whereas our study also examines a longer time period from COVID-19 diagnosis to surgery of greater than 30 days, therefore providing supplementary longer-term data than previous studies.
90-day readmission risk was not found to be significant in either analysis. This may suggest that heightened risk may be most applicable to the immediate perioperative period. More research is necessary in order to definitively discern this. Lastly, increased ASA score was found to contribute to increased 30-day complications and 90-day readmissions on all multivariable models. This is perhaps intuitive as the ASA score is intended to identify those at increased risk for perioperative complication. By this reasoning, it is, however, curious that a similar trend was not noted with regard to Charlson Comorbidity Index.
Our study has several limitations. This is a single-centered, tertiary referral center study, and further studies in larger populations across multiple institutions are needed. We performed a retrospective chart review within our institution’s EMR; therefore, it is possible that patients may have had COVID-19 testing outside of these records; however, we feel this risk is low as our EMR is highly integrated and captures the majority of data in our community. Our study is limited by the evolving nature of the COVID-19 virus and subsequent societal measures to contain it. All patients captured in the cohort were assumed to be infected with the viral strain responsible for the initial wave of the COVID-19 pandemic. Our study was conducted prior to the availability of vaccinations in order to avoid the confounding effects that different vaccines may have on altering the course of COVID-19 infection. Further studies on vaccinated populations will be necessary to examine these effects separate from the unvaccinated.
In conclusion, our study validates much of the existing literature that patients with previous COVID-19 infections carry a higher perioperative risk profile compared to COVID-naive counterparts in unvaccinated populations. This is evident through an increased 30-day complication rate, which impacts patient care on both an individual and systems-based level. As the pandemic continues to evolve, there is certainly a role for future studies to further investigate how this risk profile may change as widespread innate and vaccination-induced immunity increases and strand virulence continues to change.
References
University, Johns Hopkins. (2020) COVID 19 Dashboard. https://coronavirus.jhu.edu/map.html
Al Omar K, Bakkar S, Khasweneh L et al (2020) Resuming elective surgery in the time of COVID 19: a safe and comprehensive strategy. Updates Surg 72(2):291–295. https://doi.org/10.1007/s13304-020-00822-6
Kibbe M (2020) Surgery and COVID-19. JAMA 324(12):1151–1152
Liang ZC et al (2020) Surgical considerations in patients with covid-19 what orthopaedic surgeons should know. J Bone Joint Surg 102(e50):1–8
Collaborative COVIDSurg (2020) Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 396(27):28
Aminian A et al (2020) COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Anna Surg 272(1):e27–e29
Doglietto F, Vezzoli M et al (2020) Factors associated with surgical mortality and complications among patients with and without coronavirus disease 19 (COVID-19) in Italy. JAMA Surg 155(8):691–702
Lei S, Jiang F et al (2020) Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. Eclin Med 21:100331
Egol K, Tejwani N, Capla E et al (2005) Staged management of high-energy proximal tibia fractures. J Orthop Trauma 19(7):448–455
Kayani B, Onochie E et al (2020) The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures. J Bone Joint Surg 102-B(9):1136–1145
Gruskay J, Dvorzhinskiy A et al (2020) Universal testing for COVID-19 in essential orthopaedic surgery reveals a high percentage of asymptomatic individuals. J Bone Joint Surg 102:1379–1388
Carfi A, Bernabei R et al (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605
Tolba M, Omirah M et al (2021) Assessment and characterization of post-COVID-19 manifestations. Int J Clin Pract 75(3):e13746
El-Boghdadly K, Cook T, Goodacre T, Kua J et al (2021) SARS-CoV-2 infection, COVID-19 and timing of elective surgery. Anaesthesia 76:940–946
Collaborative, COVIDSurg (2021) Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 76:748–758
Lal B, Prasad N, Englum B, Turner D et al (2021) Periprocedural complications in patients with SARS-CoV-2 infection compared to those without infection: a nationwide propensity-matched analysis. Am J Surg 222:431–437
Baiocchi G, AguiarJr S, Duprat J, Coimbra F et al (2020) Early postoperative outcomes among patients with delayed surgeries after preoperative positive test for SARS-CoV-2: a case-control study from a single institution. J Surg Oncol 123:823–833
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: 30-Day Complication Multivariable Analysis—Severity of Infection Model
See Table
7.
Appendix 2: 30-Day Complication Multivariable Analysis—Time Between Diagnosis and Surgery Model
See Table
8.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neumaier, M., Thirukumaran, C., Ramirez, G. et al. Heightened 30-Day Postoperative Complication Risk Persists After COVID-19 Infection. World J Surg 47, 40–49 (2023). https://doi.org/10.1007/s00268-022-06767-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06767-8