Abstract
Introduction
Patients requiring total gastrectomy for gastric cancer experience a decrease in food intake leading to severe body weight loss after surgery. This loss may be prevented using a high-density liquid diet of high caloric content and minimal volume. This phase II study evaluated the feasibility and safety of a high-density liquid diet (UpLead®; Terumo Corporation, Tokyo, Japan) after total gastrectomy.
Methods
UpLead® (1 pack, 100 mL, 400 kcal/day) was administered after surgery for 28 days. The primary endpoint was the % relative dose intensity of 28 days of UpLead intake®. The secondary endpoint was % body weight loss at 1 and 3 months after surgery. The sample size was 35 considering expected and threshold values of 80 and 60%, respectively, with a one-sided alpha error of 10% and statistical power of 80%.
Results
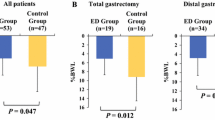
Among 35 patients enrolled before surgery between April 2018 and December 2019, 29 patients who could initiate UpLead® after surgery were analyzed. Seven patients had interrupted UpLead® intake due to taste intolerance (n = 6) and due to a duodenal stump fistula (n = 1). The remaining 22 patients completed 28 days of UpLead® intake, including temporary interruption, with no associated adverse events. The median relative dose intensity was 25.8% (95% confidence interval: 20.6–42.0%). The median body weight loss at 1 and 3 months after surgery was 7.2% (range: 3.2–13.9%) and 13.1% (range: 2.5–20.4%), respectively.
Conclusions
Oral nutritional supplementation with a high-density liquid diet (UpLead®) was safely administered but was not feasible after total gastrectomy for gastric cancer.
Clinical trial registration number UMIN000032291.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20:1–19. https://doi.org/10.1007/s10120-016-0622-4
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H (2016) Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 3(14):1286–1312. https://doi.org/10.6004/jnccn.2016.0137
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D (2014) Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 40:584–591. https://doi.org/10.1016/j.ejso.2013.09.020
Segami K, Aoyama T, Kano K, Maezawa Y, Nakajima T, Ikeda K, Sato T, Fujikawa H, Hayashi T, Yamada T, Oshima T, Yukawa N, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T (2018) Risk factors for severe weight loss at 1 month after gastrectomy for gastric cancer. Asian J Surg 41:349–355. https://doi.org/10.1016/j.asjsur.2017.02.005
Kimura Y, Nishikawa K, Kishi K, Inoue K, Matsuyama J, Akamaru Y, Tamura S, Kawada J, Kawase T, Kawabata R, Fujiwara Y, Kanno H, Yamada T, Shimokawa T, Imamura H (2019) Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann Gastroenterol Surg 3:648–656. https://doi.org/10.1002/ags3.12290
Imamura H, Nishikawa K, Kishi K, Inoue K, Matsuyama J, Akamaru Y, Kimura Y, Tamura S, Kawabata R, Kawada J, Fujiwara Y, Kawase T, Fukui J, Takagi M, Takeno A, Shimokawa T (2016) Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol 23:2928–2935. https://doi.org/10.1245/s10434-016-5221-4
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, Rino Y, Masuda M, Tsuburaya A (2013) Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 20:2000–2006. https://doi.org/10.1245/s10434-012-2776-6
Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, Yukawa N, Oshima T, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T (2017) Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol 22:476–483. https://doi.org/10.1007/s10147-017-1089-y
Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN (2013) The surgically induced stress response. JPEN J Parenter Enter Nutr 37:21S-29S. https://doi.org/10.1177/0148607113496117
Davis JL, Ripley RT (2017) Postgastrectomy syndromes and nutritional considerations following gastric surgery. Surg Clin North Am 97:277–293. https://doi.org/10.1016/j.suc.2016.11.005
Takachi K, Doki Y, Ishikawa O, Miyashiro I, Sasaki Y, Ohigashi H, Murata K, Nakajima H, Hosoda H, Kangawa K, Sasakuma F, Imaoka S (2006) Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. J Surg Res 130:1–7. https://doi.org/10.1016/j.jss.2005.08.003
Braga M, Zuliani W, Foppa L, Di Carlo V, Cristallo M (1988) Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg 75:477–480
Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa K, Mori M, Doki Y (2010) Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology 138:1312–1320. https://doi.org/10.1053/j.gastro.2009.12.058
Ida S, Hiki N, Cho H, Sakamaki K, Ito S, Fujitani K, Takiguchi N, Kawashima Y, Nishikawa K, Sasako M, Aoyama T, Honda M, Sato T, Nunobe S, Yoshikawa T (2017) Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg 104:377–383. https://doi.org/10.1002/bjs.10417
Kobayashi D, Ishigure K, Mochizuki Y, Nakayama H, Sakai M, Ito S, Kojima H, Kajikawa M, Ando M, Kodera Y (2017) Multi-institutional prospective feasibility study to explore tolerability and efficacy of oral nutritional supplements for patients with gastric cancer undergoing gastrectomy (CCOG1301). Gastric Cancer 20:718–727. https://doi.org/10.1007/s10120-016-0668-3
Akashi T, Hashimoto R, Funakoshi A (2021) Effect of a novel, energy-dense, low-volume nutritional food in the treatment of superior mesenteric artery syndrome. Cureus 13:e15243. https://doi.org/10.7759/cureus.15243
Łoś-Rycharska E, Kieraszewicz Z, Czerwionka-Szaflarska M (2016) Medium chain triglycerides (MCT) formulas in paediatric and allergological practice. Gastroenterol Rev 11:226–231. https://doi.org/10.5114/pg.2016.61374
Sripongpun P, Lertpipopmetha K, Chamroonkul N, Kongkamol C (2021) Diarrhea in tube-fed hospitalized patients: feeding formula is not the most common cause. J Gastroenterol Hepatol 36:2441–2447. https://doi.org/10.1111/jgh.15484
Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R (2009) Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 6:583–590. https://doi.org/10.1038/nrgastro.2009.148
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd (2011), English ed. Gastric Cancer 14:101–112
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K (2017) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg 265:277–283. https://doi.org/10.1097/SLA.0000000000001814
Yamada T, Hayashi T, Aoyama T, Shirai J, Fujikawa H, Cho H, Yoshikawa T, Rino Y, Masuda M, Taniguchi H, Fukushima R, Tsuburaya A (2014) Feasibility of enhanced recovery after surgery in gastric surgery: a retrospective study. BMC Surg 14:41. https://doi.org/10.1186/1471-2482-14-41
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
van Berge Henegouwen MI, van der Poll T, van Deventer SJ, Gouma DJ (1998) Peritoneal cytokine release after elective gastrointestinal surgery and postoperative complications. Am J Surg 175:311–316. https://doi.org/10.1016/s0002-9610(98)00010-5
Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A (2013) Cancer cachexia—pathophysiology and management. J Gastroenterol 48:574–594. https://doi.org/10.1007/s00535-013-0787-0
Miyazaki Y, Omori T, Fujitani K, Fujita J, Kawabata R, Imamura H, Okada K, Moon JH, Hirao M, Matsuyama J, Saito T, Takahashi T, Kurokawa Y, Yamasaki M, Takiguchi S, Mori M, Doki Y (2021) Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer 24:1150–1159. https://doi.org/10.1007/s10120-021-01188-3
Baker ML, Halliday V, Robinson P, Smith K, Bowrey DJ (2017) Nutrient intake and contribution of home enteral nutrition to meeting nutritional requirements after oesophagectomy and total gastrectomy. Eur J Clin Nutr 71:1121–1128. https://doi.org/10.1038/ejcn.2017.88
Acknowledgements
We thank all the patients, their families, investigators, and medical staff members who participated in this study.
Funding
This work is supported, in part, by Kanagawa Standard Anti-Cancer Therapy Support System (KSATTS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Yamada reports personal fees from ONO, personal fees from BMS, personal fees from Johnson and Johnson, personal fees from Taiho, personal fees from Nippon Kayaku, outside the submitted work. Dr. Oshima reports Grants and personal fees from Taiho, Grants and personal fees from Chugai, Grants and personal fees from Ono, Grants from Daiichi Sankyo, Grants and personal fees from Nippon Kayaku, Grants and personal fees from Eli Lilly, personal fees from BMS, outside the submitted work. Dr. Yoshikawa reports personal fees from Taiho, personal fees from Ono, personal fees from BMS, personal fees from Daiichi Sankyo, personal fees from MSD, personal fees from TERUMO, personal fees from Covidien, personal fees from Olympus, personal fees from Johnson and Johnson, personal fees from Chugai, personal fees from Lilly, personal fees from Nihon Kayaku, outside the submitted work. All the remaining authors have no conflicts of interest to declare.
Ethical approval
The study protocol was approved by the institutional review board of Kanagawa Cancer Center (Approval No: 2017–49). The study procedures complied with the recommendations of the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and the guidelines of the responsible Japanese governmental agency.
Informed consent
All patients were required to provide written informed consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamada, T., Hayashi, T., Fujikawa, H. et al. Feasibility and Safety of Oral Nutritional Supplementation with High-Density Liquid Diet After Total Gastrectomy for Gastric Cancer. World J Surg 46, 2433–2439 (2022). https://doi.org/10.1007/s00268-022-06639-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06639-1