Abstract
Background
Body weight loss (BWL) after gastrectomy is associated with not only a deteriorated quality of life but also a poor prognosis. Oral nutritional supplements (ONS) may be used to minimize BWL, which is observed in the first 3 months after gastrectomy and becomes stable thereafter, although the results of several randomized trials remain controversial.
Methods
We performed a multicenter, open-label randomized controlled trial including 1003 gastric cancer patients undergoing curative gastrectomy. Patients were assigned to the ONS group or the control group. In the former, 400 ml (400 kcal) per day for 12 weeks as enteral nutrition was planned, and the actual intake amount was recorded daily by patients themselves. The primary endpoint was BWL 1 year after gastrectomy.
Results
BWL data were available in 880 patients (ONS 437, control 443). BWL at 3 months was significantly lower in the ONS group than in the control group (7.1 ± 5.6% and 8.5 ± 5.8%, p = 0.0011). However, the difference gradually declined after 6 months and was not significant 1 year after surgery (9.3 ± 8.2% and 9.8 ± 8.7%, p = 0.37). In the ONS group, 50.4% of patients took more than 200 ml/day of ONS (average 301 ml) and showed significantly less BWL (8.2 ± 7.2%) at 1 year than the control (p = 0.0204).
Conclusion
The administration of ONS for 12 weeks after gastrectomy did not improve BWL at 1 year. However, the improvement in BWL remained until 1 year after surgery in patients who took more than 200 kcal/day of ONS.
Similar content being viewed by others
Introduction
Body weight loss (BWL) remains a major complication of gastrectomy. Several articles have reported that BWL is associated with not only a remarkably deteriorated quality of life (QOL), but also reduced immune function and gastric cancer patient survival [1,2,3,4]. BWL due to gastrectomy occurs due to a miscellaneous mechanism, such as hypercatabolism related to inflammatory reactions derived from surgical stress, reduced food intake and absorption owing to a loss of reservoir function, a decrease in pancreatic excretion and gastric acid level, and a reduction in ghrelin in the blood [5,6,7,8]. Despite various surgical approaches, such as avoiding total gastrectomy, [9, 10] performing minimally invasive surgery, [11] and constructing the jejunal pouch, [12] remarkable BWL remains unsolved. Although several studies of gastrectomized patients using oral nutritional supplements (ONS) have been reported, their outcomes have been inconsistent [13,14,15,16,17]. One of the reasons for these discrepancies is that there is no established ONS protocol (i.e., the type of ONS, the daily energy amount of ONS and the duration of intake are different among studies). Moreover, adherence to ONS may be another important factor that is strongly affected by nutritional counseling from surgeons, dietitians and pharmacologists.
BWL after gastrectomy is time dependent. For example, BWL that occurs after total gastrectomy has been reported to be 15 ~ 20%, in which more than 80% of BWL is observed within the first 3 months and the remaining 20% of BWL develops slowly over time [18]. This is because food intake gradually increases after gastrectomy and becomes stable after 6 months. Therefore, we hypothesized that the prevention of BWL with ONS may be sufficient for the first 3 months and that if ONS were effective, the patient would maintain the difference 1 year later.
This is the largest randomized controlled trial (RCT) of ONS ever performed, recruiting more than 1000 patients from more than 20 hospitals. The standardization of protocols and data management was achieved by meeting every 3 months. Here, we can demonstrate reliable data, which have been strongly desired among surgeons and all clinicians.
Methods
Study design and participants
A large-scale, multicenter, open-label phase III RCT in which 22 institutions in Japan participated was performed between November 11, 2013, and July 13, 2017. This RCT was organized by the Osaka University Clinical Research Group for Gastroenterological Study. We adopted the two-stage patient enrollment system, and each stage had its own criteria to omit the surgical bias derived from complications that affect the endpoints and compliance with ONS. The first-stage eligible patients were aged 20–85 years old, underwent distal (including pylorus preservation), proximal, or total gastrectomy (DG, PG, and TG, respectively) for histologically proven primary gastric cancer with no clinical distant metastasis (H0, P0, and M0 except for cy1), and had adequate organ function. Another key criterion was an Eastern Cooperative Oncology Group performance status of 0–2. The exclusion criteria were remnant gastric cancer; contraindications for Racol® NF (Otsuka Pharmaceuticals Factory, Tokyo, Japan), which was the ONS adopted in this study, and its composition was 4.38 g protein, 2.23 g lipid, and 15.62 g carbohydrate in a 100 mL volume; pregnancy; implantable medical device; and synchronous or metachronous (within 5 years) malignancies other than carcinoma in situ. After primary enrollment, patients underwent gastrectomy and postsurgical management following the routine clinical path of each hospital. Within 7 days after surgery, secondary enrollment was required on the same website according to the protocol. The secondary criteria included the following: (1) R0 or R1 (cy1) surgery, (2) no distant metastasis observed during the operation, and (3) absence of postoperative complications affecting the beginning of the oral diet, such as anastomotic leakage or pancreatic fistula. The study protocol was approved by the institutional review board of each participating hospital before initiation of the study.
Surgical procedure
Patients underwent standard gastrectomy and lymph node dissection according to the Japanese Gastric Cancer Treatment Guidelines [19]. In brief, D1 plus lymphadenectomy (D1 + dissection) was performed in patients with cT1 tumors without regional lymph node metastasis, and D2 lymphadenectomy was performed in patients with cT1 tumors with regional lymph node metastasis and in patients with cT2–4 tumors. However, D1 plus lymphadenectomy was adopted occasionally for high-risk patients even with advanced cancer depending on the situation. The surgical approach (i.e., open or laparoscopic) and reconstruction method were not prespecified. At least one expert gastric surgeon who had performed more than 100 gastrectomies involved the surgery. The operative methods and pathology results were recorded according to the 14th edition of the Japanese Classification of Gastric Carcinoma. [20] Postsurgical management, including nutritional treatments except the administration of ONS, was generally performed according to the clinical path of each participating institution.
Randomization and masking
Both the primary and secondary enrollments were performed through a web-based system established for this trial, and randomization was performed with a computer-generated minimization method. After the second enrollment, patients were randomly assigned (1:1) to either the intensive nutrition group (ONS group) or the control nutrition group (control group), and patients were stratified according to the institution, disease stage, and type of gastrectomy. Investigators were informed of the treatment allocation via the internet and performed the appropriate nutritional management. Patients and investigators were unblinded to the assignment group. The data center, based at the Osaka University Clinical Research Group, was responsible for treatment allocation, central monitoring, and statistical analyses under the supervision of the statistician in charge.
Nutritional management of each group
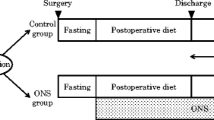
With regard to postoperative nutrition management, patients in both groups consumed a regular diet with no restrictions. Patients assigned to the ONS group received 400 ml/day (400 kcal/day) Racol® NF in addition to their regular diet for 3 months beginning within 3 days after starting a regular oral diet. In principle, the continuation of Racol® NF beyond 3 months after gastrectomy or taking another oral nutritional supplement was inhibited unless an attending physician judged its necessity. Patients assigned to the control group continued their regular diet without ONS, except when an attending physician considered the patient’s medical need for ONS. Patients recorded the amount of daily ONS intake using a prepared dietary survey notebook. Measurements of body weight were performed at baseline (preoperatively) and at 1, 3, 6, and 12 months after gastrectomy with the designated body composition analyzer [HBF-214 scale (OMRON Healthcare Co., Ltd.)]. Postoperative surveillance for recurrences with laboratory examinations and computed tomography images was planned at 6 and 12 months after surgery. Although adjuvant cancer treatment was not prescribed, S-1 treatment was started in principle if R0 resection was performed and the pathological stage was II (excluding cT1) or III according to the Japanese Gastric Cancer Treatment Guidelines [19].
Our primary endpoint was the percentage of body weight loss (%BWL) at 1 year after gastrectomy [postoperative year (POY) 1], calculated as follows: (bodyweight at baseline-bodyweight at 1 year after surgery)/bodyweight at baseline × 100. The secondary endpoints were the %BWL at 3 months after gastrectomy [postoperative month (POM) 3] and changes in nutrition-related and other blood laboratory data (lymphocyte count, hemoglobin level, C-reactive protein (CRP), total protein, albumin, total cholesterol, AST, ALT, total bilirubin, and creatinine). The severity of surgery-related complications was classified according to the Clavien-Dindo classification system and compared between the two groups [21]. With regard to other adverse events except surgical complications, the frequency and severity of those in inpatient and outpatient care were also recorded and classified into three groups: slight: temporary or minor events that need no treatment and do not affect patients’ daily lives, moderate: events that often need treatment and affect patients’ daily lives, and severe: events that need a particular intervention or hospital treatment.
Statistical analysis
We planned a total sample size of 1000 patients, and it was indispensable for 300 patients undergoing total gastrectomy to be included. This sample size would provide a power of 90% and a two-sided significance level of 0.05 to detect superiority in terms of the %BWL 1 year after gastrectomy. The %BWL was anticipated in 5.4% of patients in the ONS group and in 9.4% of patients in the control group, allowing for missing data for any reason (e.g., lost to follow-up or death) of approximately 15%. Such a statistical hypothesis was determined by reference to our past clinical trial data [22]. The planned accrual period was 4 years, and the interim analysis, which targeted the %BWL 3 months after gastrectomy, was projected at the time of enrollment with 250 patients per group. If the interim analysis could not show less body weight reduction in the ONS group than in the control group, it would lead to the termination of this trial.
We performed the statistical analysis based on a full analysis set (FAS) and a per protocol set (PPS). The FAS was defined as the group of patients who were randomized after secondary enrollment and who met the following criteria: (1) patients for whom valid data were available; and (2) patients without major protocol violations, such as informed consent procedure not being observed properly. PPS was defined as the subgroup of patients in the FAS who also met the following criteria: (1) patients who met all of the inclusion criteria and did not meet any of the exclusion criteria, (2) patients to whom nutritional therapy was administered as described above in the “Nutritional management” section and who could receive at least 50% of the total planned calories of Racol® NF by day 90 after surgery, and (3) patients in whom all parameters required to assess the primary endpoint were measured at specified time points. Continuous numerical data are expressed as the mean and standard deviation (SD), and the distribution of dichotomous data is presented as the percentage with the 95% confidence interval (CI). We used the χ2 test to compare binary variables and the t-test to compare continuous variables. All p values of less than 0.05 were judged as significant.
The primary outcome was analyzed in the FAS and PPS groups according to the protocol. Comparisons between the two groups (i.e., the ONS group and the control group) were performed using an unpaired t-test, and values in each group are documented as the mean and SD. After performing univariate regression analyses to identify factors that were potentially associated with %BWL, a multiple logistic regression analysis was performed to reveal independent factors associated with %BWL by adjusting for sex, age, body mass index (BMI), type of gastrectomy, and disease stage. We also used unpaired and paired t-tests to analyze the secondary outcomes for calculating and comparing outcomes. Subgroup analyses were performed by logistic regression analysis to assess the statistical interactions between treatment and the five similar prespecified subgroups described above. Because of the exploratory nature of subgroup comparisons, we report the test results without multiplicity adjustment of type I error. Patient characteristics were compared between the two groups using unpaired t tests or χ2 tests. The trial is registered at the UMIN Clinical Trials Registry (UMIN-CTR) (UMIN000011919).
Funding
The sponsor, the Supporting Center for Clinical Research and Education (SCCRE), which is supported by several companies, including Otsuka Pharmaceutical Factory and EN Otsuka Pharmaceutical, who sale and produce Racol® NF, had no role in the study design, data collection, analysis, interpretation, or writing of the article. The corresponding author had full access to all the data and was responsible for the decision to submit for publication.
Results
Figure 1 demonstrates the trial profile. A total of 1167 patients from 22 institutions were registered in the first stage, and the review of all case report forms revealed that three patients were ineligible for inclusion. After elective gastrectomy, 161 patients were excluded for several reasons, such as withdrawal, R2 surgery, distant metastasis, and an inability to take oral diets and medications with any surgical complications. Finally, 1003 patients were enrolled at the second stage and randomly assigned: 503 to the control group and 500 to the ONS group. Almost 70 patients in each group had no body weight data for several reasons, such as neglecting to weigh patients, withdrawals, deaths, and termination of the protocol treatment judged by surgeons due to any medical or surgical events until 3 months after gastrectomy; thus, the full analysis dataset population comprised 443 patients in the control group and 437 in the ONS group at 1 year after gastrectomy.
The patients’ baseline demographics, clinical characteristics, and surgical factors were balanced between the two groups at secondary enrollment (Table 1). Regarding 300 patients who underwent TG, 152 and 148 patients were allocated to the control and ONS groups, respectively. Except for TG, more than 90% of the patients underwent DG (320 and 321, respectively), and the remaining patients underwent PG (31 and 31, respectively). The proportion of patients who underwent a laparoscopic approach reached three-fifths. After secondary enrollment, the occurrence of all surgical complications, which consisted of hemorrhage, anastomotic leakage, pancreatic fistula, intraabdominal abscess, ileus, bile leakage, wound-related complications, pneumoniae, pleural effusion, chylous ascites, coagulation/thrombosis, and other minor complications, was 13.5% (119/880). No difference with regard to the occurrence of complications was observed between the two groups [control: 14.0% (62/443), ONS: 13.0% (57/437), p = 0.797], even for infectious complications (control: 6.5%, ONS: 6.6%). Over grade III postoperative complications according to Clavien-Dindo classification were also not different between the two groups (Supplementary Table 2). Figure 2 shows the proportion of the actual intake dose of ONS. Daily records of ONS were available in 385 of 437 patients in the FAS analysis, and the mean compliance with ONS was almost half of the total planned intake, which was 208 ± 118 (95% CI – 196–220) kcal/day.
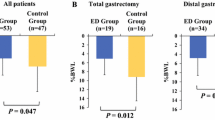
The mean ± SD %BWL at POM 3 was 8.5 ± 5.8% (95% CI 9.0–7.9) in patients in the control group and 7.1 ± 5.6% (95% CI 7.7–6.6) in patients in the ONS group; the %BWL differed significantly between the two groups (p = 0.0011), and these significant differences also continued until POM 6 [9.7 ± 7.4% (95% CI 10.4–9.0) in patients in the control group and 8.6 ± 7.2% (95% CI 9.2–7.9) in patients in the ONS group, p = 0.0228]. Moreover, the %BWL at POY 1 did not differ significantly between the two groups [control group: 9.8 ± 8.7% (95% CI 10.7–9.0) vs. ONS group: 9.3 ± 8.2% (95% CI 10.1–8.6), p = 0.37] (Fig. 3). The stratified analysis at POM 3 revealed that the %BWL was significantly lower in the ONS group than in the control group among patients who underwent DG (n = 257 and 259, respectively; 5.4 ± 4.7% [95% CI 6.0–4.8] vs. 6.7 ± 5.5% [95% CI 7.3–6.0], p = 0.0056). A similar trend was observed in patients with PG (n = 26 and 25, respectively; 7.9 ± 5.2% [95% CI 10.0 to 5.8] vs. 11.1 ± 4.8% [95% CI 13.0 to 9.1], p = 0.029), while such a trend was not shown in TG (n = 110 and 120, respectively; 11.0 ± 5.7% [95% CI 12.1–9.9] vs. 11.8 ± 4.8% [95% CI 12.7–10.9], p = 0.250) (Supplemental Figure A). In the other stratified analysis at POM 3 with regard to disease stage, the ONS group showed significantly less %BWL than the control group, but only in patients with pathological stage I (n = 256 and 265, respectively; 6.1 ± 4.9% [95% CI 6.7–5.5] vs. 7.8 ± 6.0% [95% CI 8.6–7.1], p = 0.0005), although a significant difference was not observed in patients with pStage II-III and IV (Supplemental Figure B). In terms of the %BWL at POY 1, all subgroup analyses demonstrated no significant differences between the two groups. Univariate and multivariate analyses performed to identify predictors of %BWL at POY 1 are shown in Table 2. A BMI over 22 and TG were identified as independent risk factors for BWL at POY 1. In addition, we performed a post hoc subset analysis to identify potential interactions between the %BWL at POY 1 and patient backgrounds (Fig. 4); however, no interaction was observed. Figure 5 demonstrates the body weight changes of the three groups: the control group, the PPS group (taking more than 200 kcal of Racol® per day), and the remaining ONS group (taking less than 200 kcal of Racol® per day). The number and rate of patients who fulfilled the criteria for the PPS group among the ONS group were 194 and 49.6%, respectively. The %BWL of the PPS group was significantly less at every point than that of the other two groups, which indicated that the PPS analysis had met both the primary and secondary endpoints related to the %BWL.
Subgroup analyses of the potential interaction between the %body weight loss at 1 year after gastrectomy and patient backgrounds. The data cutoff was 9.2%, which was the median value at 1 year after gastrectomy in the FAS. The odds ratio was calculated for patients in the intervention group and for those in the control group
Comparison of body weight changes after gastrectomy between the per protocol set and the other groups. Asterisks indicate a significant difference between the per protocol set and the control group (p < 0.05). Daggers indicate a significant difference between the per protocol set and the remaining intervention group (p < 0.05)
No significant differences between the two groups were observed in terms of hematological and biochemical parameters (Supplementary Table 3). We did not identify any severe adverse events directly related to the intake of ONS, although more gastroenterological adverse events, such as nausea, diarrhea, and abdominal pain, were reported in the ONS group than in the control group due to the occurrence of ONS-related adverse events (Supplementary Table 4).
Discussion
A nonvolitional reduction in body weight after gastrectomy is a phenotype associated with malnutrition, an inevitable and serious problem that correlates with a decline in postoperative QOL, decreased immune functions, and a poor gastric cancer prognosis [3, 23,24,25]. Various approaches against pleural harmful effects on body weight due to gastrectomy have been attempted, although remarkable BWL corresponding to a severe grade of malnutrition according to the Global Leadership Initiative on Malnutrition (GLIM) criteria [26, 27] remains an unresolved problem, especially for patients undergoing total gastrectomy. Whether nutritional support with ONS can be effective “universally” is of concern, since any nutritional intervention approach is indispensable for patients undergoing gastrectomy. Unfortunately, our results of the first large-scale RCT did not show that the administration of ONS for 3 months after gastrectomy could prevent BWL at POY 1 after gastrectomy as a primary endpoint, although its effectiveness was statistically significant until 6 months after gastrectomy. However, the PPS analysis demonstrated a significantly smaller reduction in BWL at both 3 months and 1 year after gastrectomy. These results may indicate that drinking more than 200 kcal/day of ONS within the first 3 months could be effective and that such efficacy could be maintained up to 1 year after gastrectomy, as we hypothesized.
Two independent risk factors related to %BWL at POY 1, namely, a high BMI (≥ 22) and TG, were extracted from the multivariate analysis, and the results were almost consistent with those from previous cohort studies [4, 16, 18]. For patients with such risk factors, receiving strong and compelling nutritional support, such as ONS and feeding tubes, may be required to prevent severe BWL.
The European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines state that early enteral feeding is relevant for any surgical patient with nutritional risks, especially those undergoing upper gastrointestinal surgery [28]. Although several clinical trials have reported the usefulness of nutritional intervention, including ONS, for short-term results (e.g., a postoperative recovery reduction in the infection ratio and a short hospital stay), the mid- and long-term effects or strategies with regard to BWL have not yet been fully discussed. Recently, gastric cancer surgeons have paid attention to short- and mid-term BWL after gastrectomy since it could affect the dose intensity of adjuvant chemotherapy and the survival of gastric cancer patients. Although some studies on ONS have been performed, the efficacy of ONS is very controversial. Although an existing positive effect of ONS on BWL may be too weak to show a significant impact even in prospective randomized studies, [29] almost all studies on ONS with patients undergoing gastrectomy have referred to the importance of adherence to ONS. Kobayashi et al. [30] reported the efficacy of postoperative ONS in a single-arm study, which was similar to our study. Coincidentally, the average dose intensity of Racol® NF in their study was 52.7% (95% CI 46.5–58.8%), with 211 kcal, and patients who tolerated more than 200 kcal of ONS showed less BWL than those reported in other studies, as in ours [30]. Compared to these studies, only previous RCTs with positive results, such as the one conducted by Imamura et al. [16], reported a higher adherence of 68.7 ± 30.4% with 206 kcal. However, the comprehensive interpretation of oral nutritional supplement adherence might indicate the necessity of an intake of more than 200 kcal of ONS per day from both previous studies and ours on ONS. Although another key point with regard to the administration of ONS is the way of increasing the adherence of ONS and the dosing period, sufficient evidence is not available, and further studies are warranted.
Our hypothesis and rationale for determining the duration and amount of oral nutritional supplement administration were based on the results of our previous randomized controlled study [22] and other reports [18]. Especially regarding the amount of caloric intake derived from ONS, we set the daily dose by converting the expected %BWL in the hypothesis to the intake energy. In addition, we hypothesized that it was the most effective to initiate ONS as soon as possible and continue for at least 3 months after gastrectomy. Although our hypothesis was that the early prevention of weight reduction during the most severe period would have a positive effect on the final body weight, it was proven incorrect with this large-scale RCT. However, routine, sufficient intake of ONS (more than 200 kcal per day) and a regular diet significantly prevented BWL not only during the treatment period, but also during the period after the termination of ONS (at 6 POM and 1 POY) (Fig. 5). This novel result might indicate that sufficient intake of ONS has a legacy effect on the prevention of BWL, which could be very useful for establishing postoperative nutritional guidelines in the clinical pathway after surgery for gastric cancer. Our study data, as a whole, indicated that the administration of ONS can be considered or recommended as a standard supportive treatment after gastrectomy, though it may be desirable to take more than 200 kcal of ONS per day.
This study had several limitations. First, we had no information on the precise caloric intake of the regular diet, and the amount of oral nutritional supplement intake was calculated by self-enumeration with a prepared dietary notebook. Therefore, the influence of oral nutritional supplement intake on the patient’s regular diet and the difference in total dietary energy intake between the two groups are unknown. Some patients may have suffered from postgastrectomy symptoms, such as feelings of fullness, abdominal discomfort, or diarrhea, due to drinking ONS. Second, all participants were Japanese, and safety and efficacy data may be applicable only to East Asians. Third, as the nutritional agent, we adopted only Racol® NF, which is only commercially available in all participating institutions for both inpatient and outpatient settings, contains the three major nutrients balanced in accordance with dietary reference intakes for Japanese patients, and prohibits other types of ONS. For gastrectomized patients, the optimal composition or configuration (e.g., an elemental diet or predigested nutrients) has been unknown from past evidence. Although the type of ONS may have little impact on the %BWL compared to the amount of calorie intake, comparative or unregulated trials with regard to oral nutritional supplement types are still required to identify the most appropriate way to use ONS. Fourth, we did not directly compare QOL at POM 3. Although the ONS group showed a significant reduction in BWL at POM 3 in the FAS, it must be discussed whether the difference of 1.4%, which corresponded to approximately 1 kg as the restraint quantity, has clinical significance for patients. It will be important to determine whether the amount of BWL can have a negative effect on patient QOL, and its answer must be able to be quantified when nutritional intervention will be performed.
Conclusion
This large-scale RCT did not show the efficacy of the routine administration of ONS for 3 months after gastrectomy for preventing BWL at POY 1, although their effectiveness was validated as an improvement over a regular diet, but only while taking them. However, a daily sufficient amount of ONS for 3 months after gastrectomy may have potential advantages and legacy effects on the reduction in BWL beyond the time of oral administration.
References
Kong H, Kwon OK, Yu W. Changes of quality of life after gastric cancer surgery. J Gastric Cancer. 2012;12:194–200.
Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc Biol Sci. 2003;270:905–11.
Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9.
Tanabe K, Takahashi M, Urushihara T, et al. Predictive factors for body weight loss and its impact on quality of life following gastrectomy. World J Gastroenterol. 2017;23:4823–30.
Friess H, Bohm J, Muller MW, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341–7.
Bragelmann R, Armbrecht U, Rosemeyer D, Schneider B, Zilly W, Stockbrugger RW. Nutrient malassimilation following total gastrectomy. Scand J Gastroenterol Suppl. 1996;218:26–33.
Kurokawa Y, Sasako M, Sano T, et al. Functional outcomes after extended surgery for gastric cancer. Br J Surg. 2011;98:239–45.
Doki Y, Takachi K, Ishikawa O, et al. Ghrelin reduction after esophageal substitution and its correlation to postoperative body weight loss in esophageal cancer patients. Surgery. 2006;139:797–805.
Furukawa H, Kurokawa Y, Takiguchi S, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. 2018;21:500–7.
Jiang X, Hiki N, Nunobe S, et al. Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer. 2011;14:194–9.
Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14:144–9.
Ward MA, Ujiki MB. Creation of a jejunal pouch during laparoscopic total gastrectomy and Roux-en-Y esophagojejunostomy. Ann Surg Oncol. 2017;24:184–6.
Aoyama T, Yoshikawa T, Ida S, et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J Cancer. 2019;10:1070–6.
Hatao F, Chen KY, Wu JM, et al. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch Surg. 2017;402:203–11.
Ida S, Hiki N, Cho H, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017;104:377–83.
Imamura H, Nishikawa K, Kishi K, et al. Effects of an oral elemental nutritional supplement on postgastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol. 2016;23:2928–35.
Kong SH, Lee HJ, Na JR, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery. 2018;164:1263–70.
Davis JL, Selby LV, Chou JF, et al. Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol. 2016;23:1639–45.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20: 1–19.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–12.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Hirao M, Takiguchi S, Imamura H, et al. Comparison of billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591–7.
Aoyama T, Sato T, Maezawa Y, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22:476–83.
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9:315–25.
Ohkura Y, Haruta S, Tanaka T, Ueno M, Udagawa H. Effectiveness of postoperative elemental diet (Elental®) in elderly patients after gastrectomy. World J Surg Oncol. 2016;14:268.
Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9.
Jensen GL, Cederholm T, Correia M, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43:32–40.
Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–50.
Koller M, Schutz T, Valentini L, Kopp I, Pichard C, Lochs H. Outcome models in clinical studies: implications for designing and evaluating trials in clinical nutrition. Clin Nutr. 2013;32:650–7.
Kobayashi D, Ishigure K, Mochizuki Y, et al. Multi-institutional prospective feasibility study to explore tolerability and efficacy of oral nutritional supplements for patients with gastric cancer undergoing gastrectomy (CCOG1301). Gastric Cancer. 2017;20:718–27.
Acknowledgements
The authors are grateful to the members of the Data Center in this study: Yoshitomo Yanagimoto for protocol production and data management; Masaaki Yamamoto, Atsushi Gakuhara, and Takeo Hara for data management; and Yuriko Takeda for data collection and management. Data collection and the electronic data capture (EDC) system were supported by Medical Edge, Tokyo, Japan.
Funding
This study was partly funded by the sponsor, the Supporting Center for Clinical Research and Education (SCCRE), which is supported by several companies, including Otsuka Pharmaceutical Factory and EN Otsuka Pharmaceutical, who sale and produce Racol® NF, and had no role in the study design, data collection, analysis, interpretation, or writing of the article.
Author information
Authors and Affiliations
Consortia
Contributions
YM: conception and design of the study; generation, collection, assembly, analysis and/or interpretation of data; and drafting and revision of the manuscript; approval of the final version of the manuscript, TO, KF, JF, RK, HI, KO, J-HM, MH, JM: generation, collection, assembly, TS, TT: conception and design of the study; generation, collection, assembly, YK: conception and design of the study; generation, collection, assembly, analysis and/or interpretation of data; MY: conception and design of the study; generation ST: conception and design of the study; generation MM: revision of the manuscript, approval of the final version of the manuscript, YD: conception and design of the study; generation, assembly, interpretation of data; revision of the manuscript; approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YD has received lecture fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Eli Lilly, MSD, Daiichi Sankyo, Yakult Honsha, Takeda Pharmaceutical, Kaken Pharmaceutical, Abbott Japan, Eisai, Shionogi, Otsuka Pharmaceutical, Ajinomoto Pharmaceutical, Teijin Pharma, Sanofi, Astellas Pharma, Tsumura, AstraZeneca, Asahi Kasei Pharma, Medtronic, Johnson & Johnson, Olympus, and Intuitive Surgical, Nippon Kayaku, Novartis Pharma, Pfizer Japan, CSL Behring, and Nestle. JM has received lecture fees from Chugai Pharmaceutical.
Human rights statement
Alinfol procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from all patients for inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyazaki, Y., Omori, T., Fujitani, K. et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer 24, 1150–1159 (2021). https://doi.org/10.1007/s10120-021-01188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01188-3