Abstract
Background
This systematic review explored the efficacy of different pain relief modalities used in the management of postoperative pain following pancreatoduodenectomy (PD) and distal pancreatectomy (DP) and impact on perioperative outcomes.
Methods
MEDLINE (OVID), Embase, Pubmed, Web of Science and CENTRAL databases were searched using PRISMA framework. Primary outcomes included pain on postoperative day 2 and 4 and respiratory morbidity. Secondary outcomes included operation time, bile leak, delayed gastric emptying, postoperative pancreatic fistula, length of stay, and opioid use.
Results
Five randomized controlled trials and seven retrospective cohort studies (1313 patients) were included in the systematic review. Studies compared epidural analgesia (EDA) (n = 845), patient controlled analgesia (PCA) (n = 425) and transabdominal wound catheters (TAWC) (n = 43). EDA versus PCA following PD was compared in eight studies (1004 patients) in the quantitative meta-analysis. Pain scores on day 2 (p = 0.19) and 4 (p = 0.18) and respiratory morbidity (p = 0.42) were comparable between EDA and PCA. Operative times, bile leak, delayed gastric emptying, pancreatic fistula, opioid use, and length of stay also were comparable between EDA and PCA. Pain scores and perioperative outcomes were comparable between EDA and PCA following DP and EDA and TAWC following PD.
Conclusions
EDA, PCA and TAWC are the most frequently used analgesic modalities in pancreatic surgery. Pain relief and other perioperative outcomes are comparable between them. Further larger randomized controlled trials are warranted to explore the relative merits of each analgesic modality on postoperative outcomes with emphasis on postoperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative pain after pancreatic resections is frequent [1, 2]. This may be attributed to a high incidence of preoperative pain resulting in use of analgesics prior to surgery and resection requiring extensive abdominal dissection with big incisions [2]. Inadequate pain control following any surgical procedure increases overall morbidity, hospital stay and recovery time [3, 4].
Epidural analgesia (EDA) is generally the analgesic modality of choice in pancreatic surgery and has been shown to have lower systemic complications such as pneumonia [5, 6], acute coronary syndrome, thromboembolism and renal failure[7], though at the expense of the need for vasopressors [8], excessive fluid administration [5], lengthened intensive care stay [9], and higher rates of complications such as postoperative pancreatic fistula (POPF) [8]. When EDA cannot be used, opioids via patient controlled analgesia (PCA) is often the preferred alternative. Unlike EDA, PCA does not promote hypotension, so undoubtedly is associated with a reduced need for vasopressor therapy and fluid administration [5]. More recently, transabdominal wound catheters (TAWC) have become more common in general abdominal surgery, as it has shown to be comparable to EDA in terms of pain relief with fewer complications, such as block failure and hypotension [10].
Although a variety of pain modalities have been explored for the management of postoperative pain after pancreatic surgery, the literature is generally limited to pair-wise comparisons, small study sizes and heterogeneity in their study population[2, 5, 8, 9, 11,12,13,14,15,16,17,18,19,20,21,22,23] making it difficult to justify routine use of one pain modality over the other. This is reflected in the recently published ERAS guidance [6] which recommends EDA for postoperative pain relief and TAWC as an alternative, however the majority of evidence for this recommendation was extrapolated from non-pancreatic surgery.
The present meta-analysis and systematic review therefore aimed to summarize and compare the efficacy of different local and regional pain relief modalities in the management of postoperative pain following pancreatic resection.
Methods
The study protocol was registered on PROSPERO (ID: CRD42020215886).
Literature search
MEDLINE (OVID), Embase, Pubmed, Web of Science and CENTRAL databases were searched from inception to September 2020, in accordance to the PRISMA framework [24]. The following query words were used: “pancreatectomy” OR “pancreatic resection” OR “pancreas surgery” OR “pancreas operation” OR “pancreatic enucleation” OR “pancreaticoduodenectomy” AND “analgesia” OR “anaesthesia” OR “pain control” OR “pain management” OR “postoperative pain” OR “neuroaxial” OR “narcotic” OR “opioid” OR “adjuvant” OR local/regional analgesic methods such as epidural analgesia, patient controlled analgesia, wound catheter, TAP blocks, spinal and intrathecal blocks. “Explode” and MeSH functions were used where appropriate. The search was limited to English literature.
Inclusion and exclusion criteria
Randomised controlled trials (RCTs) and cohort studies were included if they compared two or more local or regional analgesic methods following pancreatoduodenectomy (PD) and distal pancreatectomy (DP). To qualify for inclusion in the meta-analysis, comparable studies needed to evaluate the efficacy of analgesia using a Numerical Rating Scale (NRS) or something similar, such as the Visual Analogue Score (VAS) or compare other perioperative outcomes. Studies that did not have comparable pain scores or other perioperative outcomes were included in the narrative systematic review. Where possible the pain modalities for PD and DP were evaluated separately. Studies which included minimally invasive cases (laparoscopic or robotic) or grouped different types of surgeries or pancreatic resections together were excluded, unless subgroup analysis was available.
Data extraction
All titles and abstracts were screened independently by two authors (NA, DJ), followed by a list of articles for full text review. Relevant data was extracted and reviewed by a third author (SP). Manual screening of the reference lists in identified articles was conducted for additional papers. Authors were contacted in cases of missing data.
Primary and secondary outcome measures
The primary outcome measures were pain scores on postoperative day 2 (POD2) and day 4 (POD4) and respiratory morbidity (pneumonia). These PODs were chosen as they were the most common days when pain scores were reported allowing a statistical comparison. Pain scores were rated on the NRS from 0–10, where 0 indicated no pain at all and 10 correlated to the worst pain possible. In articles that used VAS, these were converted to the corresponding number on the NRS [25]. The secondary outcome measures included operation time (OT), bile leak, delayed gastric emptying (DGE) [26], POPF [27], length of hospital stay (LOS), mortality and opioid use (in oral morphine equivalents (OME) or milligram morphine equivalents (MME)).
Definitions
Pancreatectomy included open PD and DP. TAWC included transverse abdominis plane (TAP) block and quadratus lumborum (QL) block when the catheter was left in to administer post-operative pain relief and paravertebral catheter. Operating time was defined as including both anaesthetic time and duration of surgery.
Statistical analysis
The meta-analysis was conducted in its entirety with the packages: tidyverse [28], meta [29], metaphor [30], and MetaAnalyser (Jack Bowden and Christopher Jackson, UK) 31] in R project (R Foundation for Statistical Computing, Austria 2014). A Mantel–Haenszel random effects model was utilized to perform the pairwise meta-analysis with a Hartung– Knapp adjustment. Outcomes that had 3 or more studies with an incidence of greater than 0 were included in the analysis. Where possible, outcomes from randomised and non-randomised studies were reported separately. Primary and secondary outcomes were presented using odds ratio (OR) for categorical data and standardised mean difference (SMD) for continuous data, accompanied by respective 95% confidence intervals (CI). A p value of <0.05 was considered significant. Heterogeneity was assessed using the I2 statistic; a threshold of 50% suggested moderate heterogeneity and 75% indicated substantial heterogeneity [32].
Assessment of study quality
The quality of RCTs was evaluated using the Cochrane Risk-of-Bias tool 2.0 [33]. The Newcastle–Ottawa Scale (NOS) [34] was utilised to assess the quality of non-randomised studies.
Results
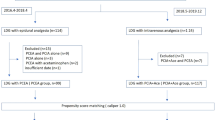
The original search identified 4912 studies, of which twenty-six full text articles were screened. Following this, twelve studies [5, 8, 13, 15, 17,18,19,20,21, 35,36,37] met the inclusion criteria, of which eight studies [5, 13, 19,20,21, 35,36,37] were included in the meta-analysis. The remaining four studies [8, 15, 17, 18] were included in the narrative review (Fig. 1).
Overall, 1313 patients were included. This incorporated five RCTs [5, 8, 15, 17, 21] and seven retrospective cohort studies [13, 18,19,20, 35,36,37], published between 2020 and 2008. Studies were conducted in the USA (n=6), UK (n=1), New Zealand (n=1), Netherlands (n=1), Korea (n=1), Italy (n=1) and other parts of Europe (n=1) (Table 1).
Eight articles compared EDA and PCA [5, 13, 19,20,21, 35,36,37] and were included in the quantitative analysis. Other articles compared EDA and TAWC [8, 15] (n=2), high dose PCA and low dose PCA (n=1) [17] and functional EDA and prematurely aborted EDA (n=1) [18]. Of these, none of the included studies stated the use of an ERAS pathway in their methods section. Where applicable, two out of four studies defined POPF and one out of four studies defined DGE using the ISGPS definitions.
EDA versus PCA
Pancreatoduodenectomy
Eight studies (1004 patients) compared EDA and PCA in patients undergoing PD, of which two studies were RCTs and the remaining were retrospective cohort studies.
Four studies [5, 13, 20, 37] reported comparable pain scores on POD2 including a total of 565 patients (EDA: n=358, PCA: n=207) and found no significant difference in pain scores between EDA and PCA (SMD 0.29, 95% CI 0.83 to 0.24, p=0.19). Subgroup analysis of non-randomised studies also found no significant difference in pain scores (SMD 0.33, 95% CI 1.36 to 0.69, p=0.3) (Fig. 2a). Subgroup analysis of randomised studies was not possible. Three studies [5, 13, 20] reported pain scores on POD4 including a total of 463 patients (EDA: n=271, PCA: n=192). There was no significant difference in pain scores between EDA and PCA (SMD 0.08, 95% CI 0.26 to 0.06, p=0.18) (Fig. 2b). Subgroup analysis of non-randomised and randomised studies was not possible. On the contrary, two other studies showed lower pain scores with EDA compared to PCA on POD 1 [21] and 2 [19] respectively.
Four studies reported incidence of pneumonia in a total of 663 patients (EDA: n=458, PCA: n=205) and found no significant difference between EDA and PCA (OR 0.43, 95% CI 0.01 to 25.33, p=0.42). Subgroup analysis of non-randomised studies also found no significant difference (OR 0.56, 95% CI 0.08 to 4.11, p=0.46) (Fig. 2c). Subgroup analysis of randomised studies was not possible. Pratt et al. [20] also reported no significant difference in pneumonia between EDA and PCA (p=0.63).
There was no significant difference in POPF (OR 0.83, 95% CI 0.54 to 1.29, p=0.22) (figure supplementary (S)1a), LOS (SMD 0.09, 95% CI -0.25 to 0.42, p=0.38) (figure S1b), bile leak (OR 1.00, 95% CI 0.32–3.14, p=0.99) (figure S1c) or DGE (OR 0.89, 95% CI 0.13–6.12, p=0.82) (figure S1d) between EDA and PCA. Subgroup analysis of non-randomised and randomised studies was not possible. There was no significant difference in mortality (OR 0.79, 95% CI 0.29–2.16, p=0.55) (figure S1e) between EDA and PCA). Subgroup analysis of non-randomised studies also found no significant difference in mortality (OR 0.84, 95% CI 0.14–5.19, p=0.85. Subgroup analysis of randomised studies was not possible.
Distal pancreatectomy
Kim et al. compared EDA with PCA in those that underwent DP (total: 42, EDA: 24, PCA: 18) and found no significant difference in pain scores on POD2 (p=0.25), POD4 (0.53), pneumonia (p=0.43), POPF (p=0.57), DGE (no incidence) or LOS (0.99).
EDA versus TAWC
Pancreatoduodenectomy
Two RCTs compared EDA with TAWC [8, 15] following PD, including a total of 84 patients. The data was not suitable for a meta-analysis; hence a descriptive analysis of outcomes was undertaken. Hutchins et al. (total: n=48, EDA: n=23, paravertebral catheter: n=25) found no significant differences in median pain scores on POD2 (p=0.93) or POD4 (p=0.44). Mungroop et al. (total: n=36, EDA: n=18, TAWC: n=18) reported similar mean pain scores on POD2 (EDA: 1.2±1.1), TAWC: 0.75±1.5) P=0.30. Hutchins et al. found no significant difference in OT (p=0.92), LOS (p=0.54) or total opioid requirements in MME (p=0.40). Mungroop et al. found no difference in mortality (p=1.0).
High dose PCA versus low dose opioid PCA
Pancreatoduodenectomy
Koo et al. [17], including a total of 110 patients, compared high dose remifentanil via PCA±ibuprofen (HR and HRI) and low dose remifentanil via PCA±ibuprofen (LR and LRI) following PD. There was no significant difference (p>0.05) in mean pain scores on POD 2 (HR: 5.2, LR: 4.9, HRI: 3.8, LRI: 4.9). No other perioperative outcomes were available.
Functional epidural versus aborted epidural
Pancreatoduodenectomy
Patel et al. [18] compared functional EDA and aborted EDA following PD including a total of 73 patients. There was no data on postoperative pain scores or pneumonia, however there was no difference in LOS (functional: n=1.9 days, aborted n=2.7 days, p=0.48).
Heterogeneity and risk bias
The outcomes to assess pain score on POD2 illustrated moderate heterogeneity. Five RCTs were assessed using the Cochrane Risk-of-Bias tool 2.0 (table S1). One study was assessed as having low risk, two as having some concerns and one as high risk. Seven cohort studies were assessed using the NOS scale (table S2). The average score was 7 stars. All studies scored 0 in the ‘comparability’ section which looked at comparability of cohorts based on the design or analysis. This was mainly attributed to the studies not matching their study groups.
Discussion
The present systematic review and meta-analysis of postoperative pain management in pancreatic surgery has demonstrated that EDA provides similar level of postoperative pain relief when compared to PCA on POD2 and POD4 after both PD and DP. Furthermore, there were no significant differences in pain relief or other perioperative outcomes when comparing EDA and TAWC, high dose PCA and low dose PCA or functional EDA and aborted EDA in PD.
EDA is widely accepted as the gold standard for pain relief following major abdominal surgery [6]. However, a recent meta-analysis of RCT’s of EDA in major abdominal surgery has shown that although EDA may provide superior pain control, the perioperative outcomes are comparable to other forms of analgesia such as PCA [38]. Furthermore, patients on EDA require increased perioperative fluid administration due to sympathetic blockade [5], and have an increased incidence of perioperative complications, particularly higher POPF rate in those undergoing PD in several recent studies [8, 39,40,41]. In the present review, when EDA was compared with PCA, pain scores were comparable and both groups had similar postoperative complications. Although comparable data was not available on the use of postoperative fluid requirement, Klotz et al. [5] in a RCT comparing EDA with PCA showed significant weight gain and need for vasopressors with EDA, albeit with no significant increase in postoperative complications, in addition to higher failure rate with EDA (18.5%). Similarly, Simpson et al. [42] in a retrospective series, showed 31% of patients developed either hypotension or opioid toxicity after EDA in the postoperative period, albeit with improved pain scores compared to non-EDA. A more recent study using a goal-directed fluid restriction strategy with EDA during pancreaticoduodenectomy has shown lower rates of POPF and DGE [41]. The present evidence regarding the impact of volume of perioperative fluids and postoperative complications in pancreatic surgery is predominantly derived from retrospective studies and larger studies are warranted. ERAS society guidelines for pancreatic surgery suggest a high evidence level for superior pain control with EDA and a low evidence level for recommendation of EDA to reduce overall morbidity [6]. The results from the present meta-analysis suggest EDA and PCA provide similar levels of pain relief and morbidity postoperatively, however further studies are needed with predefined end-points to see the effect of EDA on POPF and morbidity following pancreatic surgery [6].
TAWC are increasingly being used in pancreatic surgery, given the perceived benefits of TAWC in major abdominal surgery [10, 43]. TAWC provides a similar level of pain relief as EDA and is associated with fewer complications [8, 10, 44]. Two studies in the present review compared EDA with TAWC with different primary and secondary outcomes, making interpretation of benefits of one analgesic modality over other difficult. No significant difference was found in OT, POPF, DGE, LOS, significant morbidity, mortality or opioid use. On the contrary, a study by Newhook et al. [12] found EDA resulted in lower opioid requirements compared to TAWC, however the pain scores in the postoperative period were similar between the analgesic modalities and failure rate was higher with EDA when compared to TAWC. Furthermore, on POD3 there was tendency trend for increased need for vasopressors after EDA with a higher proportion of patients with a postoperative rise in creatinine compared to baseline. The postoperative outcomes in all included studies were comparable between EDA and TAWC, findings similar to a recent RCT of EDA and TAWC in HPB surgery [8] which showed comparable pain relief with EDA and TAWC, however TAWC was associated with shorter anaesthetic time, lower mean cumulative vasopressor and opioid consumption. A post-hoc sensitivity analysis including only patients undergoing PD again showed non-inferiority of TAWC over EDA.
There are several limitations to the present review. The postoperative pain scores were assessed by few studies thereby meta-analysis was only possible for EDA versus PCA. The varied primary and secondary outcomes of included studies meant we could not undertake a meta-analysis of perioperative outcomes and was limited to a narrative review. Most the included studies were non-randomised, thus at risk of bias. Furthermore, there is paucity of data on patient related outcomes and a lack of data regarding the perceptions and preferences of patients. However, this is the first comprehensive review of analgesic management in patients undergoing pancreatic surgery comparing relative benefits for each analgesic modality.
For a practicing clinician, the present review summarized the available evidence on postoperative pain management after pancreatic surgery. The majority of evidence is centered around the use of EDA, PCA and TAWC, with comparable pain relief with all three analgesic modalities, in addition to a similar profile of postoperative complications. Depending on the availability of local expertise, all the above analgesic modalities provide adequate pain relief in the postoperative period. Nevertheless, there is still a lack of robust randomised evidence regarding the impact of increased fluid requirements with EDA and postoperative complications such as POPF, when compared to PCA or TAWC, as none of the trials were adequately powered to evaluate this. In addition, is it unknown which analgesic modality provides adequate pain relief when patients develop postoperative complications such as postoperative acute pancreatitis or POPF. Further high-powered RCTs are warranted to assess the relative merits of these analgesic modalities on not only postoperative pain, but postoperative outcomes with emphasis on patient related outcomes and quality of life, particularly in the setting of ERAS pathways. In addition, given the morbidity profile of PD and DP is different, future trials should aim to separate these two patient groups when evaluating postoperative outcomes.
References
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183(6):630–641
Rockemann MG, Seeling W, Brinkmann A et al (1995) Analgesic and hemodynamic effects of epidural clonidine, clonidine/morphine, and morphine after pancreatic surgery–a double-blind study. Anesth Analg 80(5):869–874
Chou R, Gordon DB, de Leon-Casasola OA et al (2016) Management of postoperative pain: a clinical practice guideline from the american pain society, the american society of regional anesthesia and pain medicine, and the american society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain 17(2):131–157
Rawal N (2016) Current issues in postoperative pain management. Eur J Anaesthesiol 33(3):160–171
Klotz R, Larmann J, Klose C et al (2020) Gastrointestinal complications after pancreatoduodenectomy with epidural vs patient-controlled intravenous analgesia: a randomized clinical trial. JAMA Surg 155(7):e200794
Lassen K, Coolsen MM, Slim K et al (2013) Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg 37(2):240–258
Amini A, Patanwala AE, Maegawa FB et al (2012) Effect of epidural analgesia on postoperative complications following pancreaticoduodenectomy. Am J Surg 204(6):1000–1004
Mungroop TH, Veelo DP, Busch OR et al (2016) Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): a randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol Hepatol 1(2):105–113
Boisen ML, McQuaid AJ, Esper SA et al (2019) Intrathecal morphine versus nerve blocks in an enhanced recovery pathway for pancreatic surgery. J Surg Res 244:15–22
Ventham NT, Hughes M, O’Neill S et al (2013) Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg 100(10):1280–1289
Solis-Velasco MA, Ore Carranza AS, Stackhouse KA et al (2019) Transversus abdominis plane block reduces pain and narcotic consumption after robot-assisted distal pancreatectomy. HPB (Oxford) 21(8):1039–1045
Newhook TE, Dewhurst WL, Vreeland TJ et al (2019) Inpatient opioid use after pancreatectomy: opportunities for reducing initial opioid exposure in cancer surgery patients. Ann Surg Oncol 26(11):3428–3435
Kim SS, Niu X, Elliott IA et al (2019) Epidural analgesia improves postoperative pain control but impedes early discharge in patients undergoing pancreatic surgery. Pancreas 48(5):719–725
Groen JV, Slotboom DEF, Vuyk J et al (2019) Epidural and non-epidural analgesia in patients undergoing open pancreatectomy: a retrospective cohort study. J Gastrointest Surg 23(12):2439–2448
Hutchins JL, Grandelis AJ, Kaizer AM et al (2018) Thoracic paravertebral block versus thoracic epidural analgesia for post-operative pain control in open pancreatic surgery: a randomized controlled trial. J Clin Anesth 48:41–45
Aloia TA, Kim BJ, Segraves-Chun YS et al (2017) A randomized controlled trial of postoperative thoracic epidural analgesia versus intravenous patient-controlled analgesia after major hepatopancreatobiliary surgery. Ann Surg 266(3):545–554
Koo CH, Cho YJ, Hong DM et al (2016) Influence of high-dose intraoperative remifentanil with intravenous ibuprofen on postoperative morphine consumption in patients undergoing pancreaticoduodenectomy: a randomized trial. J Clin Anesth 35:47–53
Patel A, Stasiowska M, Waheed U et al (2014) Poor analgesic efficacy of epidural analgesia in critical care patients after pancreaticoduodenectomy. Pancreas 43(3):373–379
Choi DX, Schoeniger LO (2010) For patients undergoing pancreatoduodenectomy, epidural anesthesia and analgesia improves pain but increases rates of intensive care unit admissions and alterations in analgesics. Pancreas 39(4):492–497
Pratt WB, Steinbrook RA, Maithel SK et al (2008) Epidural analgesia for pancreatoduodenectomy: a critical appraisal. J Gastrointest Surg 12(7):1207–1220
Marandola M, Cilli T, Alessandri F et al (2008) Perioperative management in patients undergoing pancreatic surgery: the anesthesiologist’s point of view. Transplant Proc 40(4):1195–1199
Gottschalk A, Freitag M, Steinacker E et al (2008) Pre-incisional epidural ropivacaine, sufentanil, clonidine, and (S)+-ketamine does not provide pre-emptive analgesia in patients undergoing major pancreatic surgery. Br J Anaesth 100(1):36–41
Rockemann MG, Seeling W, Duschek S et al (1997) Epidural bolus clonidine/morphine versus epidural patient-controlled bupivacaine/sufentanil: quality of postoperative analgesia and cost-identification analysis. Anesth Analg 85(4):864–869
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Bijur PE, Latimer CT, Gallagher EJ (2003) Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med 10(4):390–392
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surg 142(5):761–768
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surg 138(1):8–13
Wickham. H. Tidyverse. In: Easily install and load the ‘tidyverse’ [Available from: https://cran.r-project.org/web/packages/tidyverse/index.html.
Guido S (2007) Meta: an R package for meta-analysis. R News 7:40–45
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Bowden JJ, C. (2016) An interactive visualisation of meta-analysis as a physical weighing machine [Available from: https://cran.r-project.org/web/packages/MetaAnalyser/index.html.
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Sterne JAC, Savović J, Page MJ, et al. (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ.;366: l4898
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Axelrod TM, Mendez BM, Abood GJ et al (2015) Peri-operative epidural may not be the preferred form of analgesia in select patients undergoing pancreaticoduodenectomy. J Surg Oncol 111(3):306–310
Sakowska M, Docherty E, Linscott D et al (2009) A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 33(9):1802–1808
Shah DR, Brown E, Russo JE et al (2013) Negligible effect of perioperative epidural analgesia among patients undergoing elective gastric and pancreatic resections. J Gastrointest Surg 17(4):660–667
Hughes MJ, Ventham NT, McNally S et al (2014) Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg 149(12):1224–1230
Bruns H, Kortendieck V, Raab HR et al (2016) Intraoperative fluid excess is a risk factor for pancreatic fistula after partial pancreaticoduodenectomy. HPB Surg 2016:1601340
Han IW, Kim H, Heo J et al (2017) Excess intraoperative fluid volume administration is associated with pancreatic fistula after pancreaticoduodenectomy: A retrospective multicenter study. Med (Baltim) 96(22):e6893
Sulzer JK, Sastry AV, Meyer LM et al (2018) The impact of intraoperative goal-directed fluid therapy on complications after pancreaticoduodenectomy. Ann Med Surg (Lond) 36:23–28
Simpson RE, Fennerty ML, Colgate CL et al (2019) Post-pancreaticoduodenectomy outcomes and epidural analgesia: a 5-year single-institution experience. J Am Coll Surg 228(4):453–462
Mungroop TH, Bond MJ, Lirk P et al (2019) Preperitoneal or subcutaneous wound catheters as alternative for epidural analgesia in abdominal surgery: a systematic review and meta-analysis. Ann Surg 269(2):252–260
Bell R, Pandanaboyana S, Prasad KR (2015) Epidural versus local anaesthetic infiltration via wound catheters in open liver resection: a meta-analysis. ANZ J Surg 85(1–2):16–21
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akter, N., Ratnayake, B., Joh, D.B. et al. Postoperative Pain Relief after Pancreatic Resection: Systematic Review and Meta-Analysis of Analgesic Modalities. World J Surg 45, 3165–3173 (2021). https://doi.org/10.1007/s00268-021-06217-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06217-x