Abstract
Background
Donor variational arteries often require complex reconstruction.
Methods
We analysed the incidence of different variations, types of arterial reconstructions and their impact on post-operative results from 409 patients undergoing liver transplantation at Karolinska Institute between 2007 and 2015.
Results
A total of 292 (71.4%) liver grafts had a standard hepatic artery (SHA), and 117 (28.6%) showed hepatic artery variants (HAV). 58% of HAV needed reconstruction. The main variations were variant left hepatic artery (45.3%) from the gastric artery; variant right hepatic artery (38.5%); and a triple combination of variant right and left hepatic artery and the proper hepatic artery from the common hepatic artery (12.8%); other 3.4%. Patients/graft survival and arterial complications were not different between SHA and HAV. Incidence of biliary stricture was numerically higher in left hepatic artery variants (p = 0.058) and in variants where no arterial reconstruction was performed (p = 0.001). Operation and arterial warm ischaemia time were longer in the HAV group. The need for intraoperative re-reconstruction was higher in the HAV group (p = 0.04). Intraoperative bleeding was larger after back-table reconstruction than with intraoperative reconstruction (p = 0.04).
Conclusion
No overall differences were found between the HAV and the SHA groups. Occurrence of a variant left hepatic artery and HAV with no reconstruction seems to increase the risk of biliary strictures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The documented variation of hepatic artery in the population is between 31 and 49% [1]. There are several studies that define the anatomic classification systems of hepatic arteries, and Michel’s classification is used the most [2,3,4,5,6].
The presence of all hepatic arteries that are accessory or as a replacement should be demonstrated during donor liver operation or back-table work. However, that might not always be possible to determine whether an artery is replaced or accessory due to intrahepatic arterial branches which are not dissected, and angiography is not performed routinely. Once the hepatic artery variations have been recognized, it is possible to assess the necessity of arterial reconstruction to provide an optimal arterial blood supply for the graft. Hepatic artery thrombosis (HAT) is a life-threatening complication in liver transplantation and may require retransplantation. HAT presents 3–9% of all orthotopic liver transplantation (OLT) [7]. The risk factors for HAT have been identified; however, systematic analysis assessing the role of HAV and the type of reconstruction on the outcome is rare [1, 8, 9]. The quality of reconstruction of those arteries is essential to prevent arterial thrombosis, which in turn leads to biliary complications and graft loss [10,11,12,13].
The purpose of this study was to analyse the impact of the HAV and the effect of hepatic artery reconstruction on patients and grafts survival in full-sized adult OLT patients. Additionally, we analysed its effect on primary graft function, HAT and biliary complications.
Materials and methods
Analysis has been done from the data of all patients who 18 years of age or older, undergoing whole organ liver transplantation at Karolinska University Hospital in Stockholm between May 2007 and June 2015. Living donor liver transplantation, domino transplantation, multiorgan transplantation, intraoperative death, portocaval transposition and arterial interposition grafts, split and reduced grafts were excluded from the study. Furthermore, in cases of patients receiving a second liver graft within 3 years after the first transplantation, only the outcome of the first transplantation was included in the study analysis.
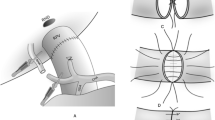
Donor arterial anatomy was recorded as it was described in operative notes from the donor operation and the recipient transplantation procedures. At that time during the study period, we did not perform routine radiological examination before organ harvesting. However, today, partially based on the result from this study we perform routinely CT liver scan in every donor in part to recognize arterial variations and to estimate liver graft volume. Cases with extra arteries, accessory or replaced, were defined as a hepatic artery variant (HAV); replaced or accessory right hepatic artery (RHA) from superior mesenteric artery (SMA) or elsewhere, Michel’s (M) type III and VI was classified as variant RHA; accessory or replaced left hepatic artery (LHA) from left gastric artery (LGA) (M type II and V), which was classified as variant (LHA); RHA from SMA + LHA from LGA + common hepatic artery (CHA) from truncus coeliacus (M type VII and type VIII), which was classified as triple; both LHA from LGA and RHA from SMA with absence of CHA, double replaced system (M type IV), which was classified as other (Fig. 1).
Arterial anastomosis was performed using 6/0 or 7/0 running or interrupted vascular sutures Prolene® with using loupe glasses in magnifications raging from 2.2 to 3.2. The reconstruction method and whether reconstruction was done during back-table work or during the recipient operation and it was chosen by the recipient surgeon under consideration of the anatomy of the graft and the anatomical arterial variation found in the recipient. The principle was to reconstruct any of the variational arteries. In the cases with appropriate artery, we used branch patch in both donor and recipient artery to perform anastomosis. If patch creation was not possible, end-to-end anastomosis was performed with running or interrupted suture based on diameter of the vessel. In the variant artery group, arterial anastomosis was performed either with a trunk patch on the graft side with one anastomosis (“no-reconstruction” group) (Fig. 2a) or used donor or recipient branch patch for extra anastomosis to reimplant variant arteries (“reconstruction” group) (Fig. 2b, c). Portal and arterial blood flow was measured intraoperatively after reperfusion with ultrasonic flow measurement (Vingmed®). The first protocol liver biopsy (zero biopsy) was taken after reperfusion of the liver graft just before closure of the abdomen. Routine Doppler ultrasound examination was performed on the first post-operative day to evaluate portal or arterial thrombosis. In the patients with questionable ultrasonographic findings and/or clinical suspicion of HAT or portal thrombosis, computed tomography angiography was performed to verify diagnosis. If normal circulation was shown in liver in the first USG examination, the next examination was performed only if there were any clinical indication, such as elevated liver blood tests. After the first examination within the first 24 h after transplantation, artery surveillance includes control Doppler ultrasound at 3-month and 1-year check-up.
Examples of hepatic artery anastomosis techniques of variant LHA from LGA and variant RHA: a arterial supply was created with one anastomosis for both, main and variant LHA arteries, using trunk on the graft side (no-reconstructed group); b additional reconstruction was made between a branch on graft main artery and variant LHA (reconstructed group); c additional reconstruction was made between a branch on graft main artery and variant RHA (reconstructed group)
Immunosupressive protocols were based on basiliximab therapy followed by tacrolimus, mycophenolate mofetil and prednisolone triple therapy. The standard thrombosis prophlaxis was 500 ml of dextran daily intravenous infusion during the first five days after transplantation followed by daily peroral 75 mg acetylsalicyl acid for a one year after transplantation.
Donor variables were age, sex, BMI, serum liver functions and sodium, days of stay at intensive care unit (ICU), cold ischaemia time (CIT) (from donor cold organ perfusion start time to recipient portal reperfusion time), arterial warm ischaemia time (from portal reperfusion to the end of the total hepatic arterial reperfusion) reason of the brain dead and rate of liver steatosis in the zero biopsy. Recipient variables tested were age, sex, BMI, indication of transplantation, MELD score, time of waiting list, warm ischaemia time (WIT) (from liver in the abdomen and portal reperfusion), days of stay at ICU, total days of hospitalization and preoperative serum transaminases as well as on post-operative days 1, 2, 3, 4, 5, 6, 7, 14 and 30. Primary graft non-function defined as UNOS definition, dead due to graft non-function and retransplantation in 10 days after transplantation, early allograft dysfunction defined as followings: alanine transaminase (ALT) and/or aspartate aminotransferase (AST) >2000 IU/l (>34 µkat/l) within the first 7 days, bilirubin ≥10 mg/dl (≥171 µmol) on day 7 [14], acute rejection up to day 30 post-transplantation, and biliary and vascular complication (arterial thrombosis, pseoudoaneurysm and portal thrombosis) were also evaluated. Biliary complications were evaluated by our local transplant registry, review of radiological assessment and patient journals. Biliary complications were divided as: non-anastomotic strictures and only anastomotic strictures, followed by ERCP or PTC intervention at least once [15]. Biliary stricture occurred within 1 year of transplantation defined as early stricture and if occurred after more than 1 year defined as late stricture. Biliary leakage was diagnosed by cholangiography or bilious secretion and considered as a primary complication.
Patient death and graft loss were included in the analysis regardless of etiology. The results were analysed in relation to the presence of a standard hepatic artery, hepatic artery variations, need for arterial reconstruction, arterial reconstruction on back table versus during recipient operation, and the location of variant arterial anastomosis. The need for perop arterial re-reconstruction after primary arterial reperfusion was also analysed.
Statistical analysis
Descriptive statistics were used for donor/recipient parameters. The data are presented as mean values ± standard deviations and median (minimum–maximum). Patients and graft survival were evaluated with the Kaplan–Meier analysis. To compare categorical variables, the Chi-square and Fisher’s exact analysis was used. Homogeneity and normality of data were evaluated with the Kolmogorov–Smirnov analysis. The Mann–Whitney U test was used to evaluate nonparametric variables. The student t test was used to evaluate parametric variables. Continuous variables among the groups were compared with the one-way analysis of variance (ANOVA). Multivariate logistic regression analysis was used to analyse of the risk factor assessment for biliary stricture. The analysis was carried out with variable which found to be statistically significant with student t test or Chi-square analysis and also on variables which had potential clinical relevance, including CIT, arterial WIT, total bleeding/transfusion, donor age and HAT. The SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL) was used to perform statistical analysis. The results were considered to be significant if p value <0.05.
Results
Between May 2007 and June 2015, 546 orthotopic liver transplantations were performed at our centre. According to the exclusion criteria, 137 patients were excluded from the study. 409 patients were included; 292 (71.4%) liver grafts had standard hepatic artery (SHA) and 117 (28.6%) presented a hepatic artery variant (HAV). Mean follow-up was 42 ± 28 months (1–105 month) for SHA group and 47 ± 28 months (3–104 month) for HAV group (p = 0.07). In the SHA group, donor mean age was 54.1 ± 15.8 years and 52.3 ± 17.6 years in the HAV group (p = 055). Donor characteristics were similar between the groups (data not shown). In the SHA group, recipient mean age was 51.6 ± 12.3 and 49.1 ± 13.5 in the HAV group (p = 0.09). Other recipient’s baseline characteristics were not different between the groups (data not shown).
The variations of graft hepatic artery were: variant LHA (M type II and V); 53 donors, 12.9%; variant RHA (M type III and VI, 45 donors, 11% (2 from GDA, 1 directly from aorta, 42 from SMA); triple artery (M type VII and type VIII) 15 donors, 3.7%; other variations (M type IV, 4 donors, 1%) (Fig. 1).
The artery used for graft main arterial reconstruction was different between the SHA and HAV groups and consisted of the following: in SHA, 7 (2.4%) using graft celiac trunk, 150 (51.7%) using graft GDA, 24 (8.3%) using graft CHA, 15 (5.2%) using graft hepatica propria, 86 (29.7%) using graft splenic artery, 8 (2.8%) using graft aortic patch. In the HAV group: 27 (23.1%) using graft celiac trunk, 12 (10.3%) using graft GDA, 15 (12.8%) using graft CHA, 14 (12%) using graft hepatica propria, 29 (24.8%) using graft splenic artery, 16 (13.7%) using graft aortic patch and 4 (3.4%) using graft SMA for main artery reconstruction (p < 0.001).
The intraoperative parameters are presented in Table 1. Type of hepatic vein reconstruction, bile duct reconstruction and use of bile duct stent were not different between the groups. The rate of perioperative artery re-reconstruction due to intraoperative arterial thrombosis, unsatisfied artery flow or kinking was 5.5% in the SHA group and 12.8% in the HAV group (p = 0.01). Signs of ischaemia on zero biopsy were not different between the two groups (p = 0.09).
Pre-transplantation and post-transplantation blood chemistry tests and post-transplantation liver enzymes were not different between the both groups (data not shown). Incidence of post-operative complications such as biliary complications, primary non-function, re-operation due to bleeding, allograft dysfunction and portal thrombosis was not different between the SHA and HAV groups. The total post-transplant arterial thrombosis rate was 0.7%. Arterial complications occurred in 3 (1%) patients (thrombosis in one patient, arterial pseudoaneurysm in two patients) in the simple artery group and in 4 (3.4%) patients (2 patients with thrombosis, and 2 with arterial pseudoaneurysm) in the variant artery group (p = 0.09). All the patients with pseudoaneurysm died due to septic complications after coiling (diffuse biliary strictures, duodenum and pancreas necrosis, intrahepatic abscesses) within 3–15 months after transplantation. Of the three patients with arterial thrombosis, one was retransplanted due to biliary complications and one underwent thrombectomy with revision of artery anastomosis and survived with good graft function and open artery. A liver abscess occurred in the third patient and was drained percutaneously. This patient died three months after transplantation due to pulmonary embolism.
The overall and 1, 12, 60 month graft survival rates were 79% and 97%, 92%, 76% in the SHA group and 86% and 99%, 95%, 84% in the HAV group, respectively (p = 0.07) (Fig. 3). The overall and 1, 12, 60 month patient survival rates were 82% and 98%, 92%, 79% in the SHA group, 86% and 100%, 96%, 85% in the variant group, respectively (p = 0.18) (Fig. 4).
Comparison of subgroups regarding arterial variants and the results of different arterial reconstructions
Comparisons of different variation of arteries are shown in Table 2. Biliary strictures showed a tendency to occur more frequently in livers with variant LHA (M type II/V) group than the other groups (p = 0.058). When comparing the two most common arterial variations (M type II/V to the M type III/VI), biliary strictures were more common in patients with variation M type II/V (p = 0.01). Post-transplantation liver blood tests were not different between the subgroups (data not shown).
In 42 liver grafts (35.9%) with HAV, no reconstruction was performed because the arterial supply was ensured by a common trunk [M type II and V in 37 donors (88.1%), M type III and VI in 4 donors (9.5%), and M type VII and VIII in 1 donor (2.4%)]. A total of 68 (58.1%) HAV which required additional arterial anastomosis reconstruction performed on back table or intraoperatively: M type III and VI in 40 donors (58.8%); M type IV in 4 donors (5.9%); M type VII and VIII in 14 donors (20.6%); M type II and V in 10 donors (14.7%), (p < 0.001). Biliary strictures, both, anaostomotic and non-anastomotic were more common in variant cases without arterial reconstruction than in cases in which reconstruction was performed (p = 0.001). This difference was confirmed by logistic regression multivariate analysis that showed the absence of reconstruction was an independent risk factor for biliary stricture (p = 0.002, OR = 8.3, 95% CI: 2.1–32.4). Additionally, donor age, leakage, total bleeding and total blood transfusion under operation were the other independent risk factor for biliary stricture.
In the LHA variant group, reconstruction was performed in 10 out of 53 (18.9%) patients. No patient in LHA variants group with reconstruction developed biliary strictures. However, in 10 (27%) patients without such reconstruction, biliary strictures of clinical significance occurred (p = 0.09).
A total of 6 patients with LHA variants and 1 RHA variant could not be repaired due to too small diameter of the artery or it was severely injured during the donor procurement (4 cases with irreversible damage from donor operation, and 3 cases with technical failure mainly due to too small vessel diameter). Biliary strictures occurred in 2 of 6 patients with ligated LHA variants and were not different when compared with no-reconstructed LHA variants [2 (33%) vs. 10 (27%) p = 0.7]. Additionally, biliary stricture was not different in reconstructed LHA variants when compared with ligated LHA variants [0 (0%) vs. 2 (33%) p = 0.12].
The arteries used for arterial reconstruction consisted of the following: 26 (38.8%) RHA to graft GDA; 10 (14.9%) RHA anastomosed to graft splenic artery; 11 (16.4%) RHA to the recipient’s GDA or right hepatic artery, 3 (4.5%) RHA to graft celiac trunk, 7 (10.4%) LHA to graft GDA, 1 (1.5%) LHA to graft splenic artery, 4 (6%) LHA to the recipient’s GDA or left hepatic artery, in two patients (3.0%) combined RHA to graft GDA and LHA to splenic artery. In 3 (4.5%) patients, arterial anastomosis was performed either as a side-to-side to the graft main artery or to a small branch on the graft main artery. Intraoperative total bleeding, total blood transfusions and cold ischaemia time were bigger and longer in patients after arterial reconstruction (Table 3).
Impact of the back-table versus intraoperative reconstruction
A total of 68 (58.1%) HAV required back-table or intraoperative additional arterial anastomosis reconstruction. Twenty were reconstructed at back table and 47 intraoperatively after portal reperfusion. In one patient, there is no clear information regarding timing of artery reconstruction. In all intraoperative reconstruction cases, reconstruction of an additional artery was performed after portal reperfusion and partial arterial reperfusion after reperfusion of the liver graft via the main artery (Table 4). Similar types of variants were seen in the two groups. Rate of preoperative artery re-reconstruction due to unsatisfied artery flow, thrombosis or kinking was higher in the intraoperative reconstruction group (p = 0.05). Intraoperative total bleeding was much more in the back-table reconstruction group (p = 0.04). Arterial WIT was longer in the intraoperative reconstruction group (p = 0.004). Overall graft/patient survival and biliary complication was similar between the two groups.
Impact of the site of arterial reconstruction, recipient or donor side
In 68 HAV, which required arterial reconstruction, 15 reconstructions were performed with the recipient’s arteries and the remaining 52 using graft arteries. In one patient, there was no information regarding the position of reconstruction. Similar types of variants were seen in the two groups. Arterial WIT, CIT, operation time, blood flow, bleeding the necessity of perop re-reconstruction, arterial complication, biliary complications and patient/graft survival were not different between groups (data not shown).
Is a graft artery with gastroduodenal artery (GDA) patch for anastomosis superior to other alternatives in patients with standard hepatic artery?
A graft artery with GDA patch was used in 150 patients. In 141 transplantations, other alternatives were used. There were no differences between two groups in term of recipient and donor demographic characteristics except for lower donor BMI in the GDA patch group (p = 0.025). In the GDA group, operation time was shorter (p = 0.003). There were no arterial flow differences between the groups, but portal flow was higher in the non-GDA group (p = 0.027). A higher post-operative bilirubin level on days 3–7 was seen in the non-GDA group (p = 0.02). There were no differences in term of graft/patient survival and biliary complications (Table 5).
Discussion
Hepatic arterial complications such as thrombosis after liver transplantation have a significant effect on mortality and morbidity [16]. It has previously been reported that donor anatomical variations of hepatic artery, which needs complex arterial reconstruction, might be associated with a higher incidence of arterial complications [17]. However, some studies have also pointed out that arterial variations do not affect the arterial or biliary complications [18]. Previous studies have also showed that liver function was not effected by arterial reconstruction [1, 10, 19]. In our series, incidence of HAV was found in 28.6% liver grafts. However, there were a longer arterial warm ischaemia time and higher rate of preoperative arterial re-reconstruction in the variant group. There were no overall differences between the variant and the standard artery groups regarding biliary and arterial complication or graft and patient survival. Interestingly, the occurrence of biliary strictures was significantly associated with presence of variant LHA. This may indicate the importance of reconstruction of a left hepatic artery variant to prevent biliary strictures. Furthermore, this is also underscored by the reduced incidence of biliary strictures in the reconstructed cases in comparison with the cases where reconstructions were judged not to be necessary.
During harvesting of organs, it is important to correctly identify the graft arterial anatomy to avoid damage and to do reconstruction planning. Soliman et al. analysed patients with hepatic artery thrombosis (HAT) after complex arterial reconstruction [17]. In their study, the presence of a left accessory artery resulted in 12% incidence of HAT compared with 7.4% with a right accessory artery. However, this difference was not statistically significant (p < 0.1). In the presented study, HAT was not different between the variant and standard artery group and total HAT incidence was low (0.7%). This could be probably due to our post-operative intensive thrombosprophylaxis procedure which can reduce the incidence of HAT [20].
Back-table reconstruction may prolong the back-table work, but the CIT is not inevitable prolonged [13]. In our study, CIT was not different between back table and intraoperative reconstruction groups. However, arterial warm ischaemia time was prolonged when variational artery reconstruction was performed during recipient operation. Artery reconstruction during back table correlates with more bleeding during the operation but lower incidence of re-reconstruction after arterial reperfusion. This did not seem to have any negative effect on outcome.
Pérez-Saborido et al. [1] showed that the presence of a graft RHA variant and the need for reconstruction was associated with longer CIT and shorter overall survival. The findings of our study did not indicate any differences between arterial variations regarding cold ischaemia time, patient and graft survival. However, we observed that biliary strictures were more common in liver with LHA variation (including 10 reconstruction on LHA and 37 reconstructions on trunk level). Incidence of biliary stricture was higher in such variant cases when no arterial reconstruction was performed because the arterial supply was ensured for both main and variant LHA by a common trunk. Absence of reconstruction was shown as an independent risk factor with regression analysis as well. Variants of LHA were less often reconstructed with an extra anastomosis when compared to other variants. The need for this may be underestimated by the possibility of having a common trunk. Reconstruction of variant LGA with an extra anastomosis with graft or recipient branch artery, such as GD, might improve the arterial supply rather than leaving a long graft artery using a common trunk for anastomosis to supply blood for both main and variant artery. Reconstruction of a variant LGA rather than leaving a long graft artery using a common trunk should be considered. Preservation of the aberrant LHA during liver transplantation is recommended; however, it may also be a risk factor for HAT if long graft artery is used to do only one anastomosis. Herrero et al. [21] presented in their study that the use of a long graft artery to create arterial reconstruction is an independent risk factor for early HAT. Montalti et al. showed in their prospective study that the incidence of non-anastomotic biliary strictures was significantly higher in cases where accessory LHA non-ligated than in the ligated group. They conclude that an aberrant LHA if demonstrated as an accessory can be safely ligated [22].
Localization of the graft to recipient arterial anastomosis is dependent on the both donor and recipient individual anatomy but also on the preference of the surgeon. A typical anastomosis in grafts with standard anatomy is between a graft GDA patch and the corresponding place in the recipient by an opening in the proper and common hepatic artery using a wide patch to side anastomosis with running sutures [23]. We compared this typical anastomosis to all other variants in standard anatomy cases and found no differences in arterial complications, graft survival or arterial flow. However, portal flow after reperfusion and post-operative first month bilirubin level was higher in the non-GDA group.
In some HAV cases, it might not be possible to use the graft artery to perform a reconstruction. In these cases, recipient right/left hepatic artery, recipient gastroduodenal artery or recipient accessory artery might be used for reconstruction. In our study, it did not affect patients/graft survival and the occurrence of complications.
The presented study has limitations: This is a retrospective study with relatively few patients with variant arteries. Variant arteries could not be investigated as if accessory or replaced because we did not perform routine radiological examination before organ harvesting in the time of study period and we did not investigate with ultrasound examination to show if there was a back flow in any of the reconstructed artery.
In conclusion, in our material, 28.6% of donor grafts presented an arterial variant. In 58% of them, reconstructions involving at least two arterial anastomoses were needed. Total incidence of HAT was 0.7%. Reconstruction seems to compensate for longer arterial warm ischaemia time and the higher preoperative artery re-reconstruction rate that occur when a variant hepatic artery is present. Reconstruction of variant LGA with an extra anastomosis with graft or recipient branch artery, such as GD, seems to be beneficial rather than leaving a long graft artery using a common trunk for anastomosis to supply blood for both main and variant artery. Graft/patient survival rates were not inferior in patients receiving HAV graft.
Abbreviations
- BMI:
-
Body mass index
- CHA:
-
Common hepatic artery
- CIT:
-
Cold ischaemia time
- GDA:
-
Gastroduodenal artery
- HAV:
-
Hepatic artery variant
- HAT:
-
Hepatic artery thrombosis
- ICU:
-
Intensive care unit
- LHA:
-
Left hepatic artery
- LGA:
-
Left gastric artery
- MELD:
-
Model for end stage liver disease
- OLT:
-
Orthotopic liver transplantation
- RHA:
-
Right hepatic artery
- SHA:
-
Standard hepatic artery
- SMA:
-
Superior mesenteric artery
- WIH:
-
Warm ischaemia time
References
Pérez-Saborido B, Pacheco-Sánchez D, Barrera Rebollo A et al (2012) Incidence of hepatic artery variations in liver transplantation: Does it really influence short and long term results? Transpl Proc 44(9):2606–2608
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220(1):50–52
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112:337–347
Rong GH, Sindelar WF (1987) Aberrant peripancreatic arterial anatomy: considerations in performing pancreatectomy for malignant neoplasms. Am Surg 53:726–729
Abdullah SS, Mabrut JY, Garbit V et al (2006) Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat 28(5):468–473
Donato P, Coelho P, Rodrigues H et al (2007) Normal vascular and biliary hepatic anatomy: 3D demonstration by multidetector CT. Surg Radiol Anat 29(7):575–582
Silva MA, Jambulingam PS, Gunson BK et al (2006) Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl 12(1):146–151
Ozsoy M, Zeytunlu M, Kilic M et al (2011) The results of vascular and biliary variations in turks liver donors: comparison with others. ISRN Surg 3:670–683
Duffy JP, Hong JC, Farmer DG et al (2009) Vascular complications of orthotopic liver transplantation: experience in more than 4200 patients. J Am Coll Surg 208(5):896–903
Hevelke P, Grodzicki M, Nyckowski P et al (2003) Hepatic artery reconstruction prior to orthotopic liver transplantation. Transpl Proc 35(6):2253–2255
Brems JJ, Millis JM, Hiatt JR et al (1989) Hepatic reconstruction during liver transplantation. Transplantation 47:403
Merion RM, Burtch GD, Ham JM et al (1989) The hepatic artery in liver transplantation. Transplantation 48:438
Melada E, Maggi U, Rossi G et al (2005) Back-table arterial reconstructions in liver transplantation: single-center experience. Transpl Proc 37(6):2587–2588
Olthoff KM, Kulik L, Samstein B et al (2010) Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 16:943–949
Buis CI, Verdonk RC, Van der Jagt EJ et al (2007) Nonanastomotic biliary strictures after liver transplantation, part 1: radiological features and risk factors for early versus late presentation. Liver Transpl 13:708–718
Marcos A, Ham JM, Fisher RA et al (2000) Surgical management of anatomic variations of the right lobe in living donor liver transplantation. Ann Surg 231:824–831
Soliman T, Bodingbauer M, Langer F et al (2003) The role of complex hepatic artery reconstruction in orthotopic liver transplantation. Liver Transpl 9(9):970–975
Soin AS, Friend PJ, Rasmussen A et al (1996) Donor arterial variations in liver transplantation: management and outcome of 527 consecutive grafts. Br J Surg 83(5):637–641
Ferraz-Neto BH, Meira-Filho SR, Hidalgo R et al (2007) Correlation between graft arterial anatomy and biliary complications after liver transplantation. Transpl Proc 39(8):2514–2515
De Pietri L, Montalti R, Nicolini D et al (2018) Perioperative thromboprophylaxis in liver transplant patients. World J Gastroenterol 24(27):2931–2948
Herrero A, Souche R, Joly E et al (2017) Early hepatic artery thrombosis after liver transplantation: What is the impact of the arterial reconstruction type? World J Surg 41(8):2101–2110. https://doi.org/10.1007/s00268-017-3989-4
Montalti R, Benedetti Cacciaguerra A, Nicolini D et al (2018) Impact of aberrant left hepatic artery ligation on the outcome of liver transplantation. Liver Transpl 24(2):204–213
Meneu-Diaz JC, Moreno-Gonzalez E, Garcia Garcia I et al (2004) Hepatic allograft arterialization by means of the gastroduodenal bifurcation (branch patch) as a prognostic factor. Transplantation 77(10):1513–1517
Acknowledgements
Open access funding provided by Karolinska Institute. We thank Dr. John Sandberg M.D for drawing the new Fig. 2a/b/c.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee for Clinical Studies at Karolinska University Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karakoyun, R., Romano, A., Yao, M. et al. Impact of Hepatic Artery Variations and Reconstructions on the Outcome of Orthotopic Liver Transplantation. World J Surg 44, 1954–1965 (2020). https://doi.org/10.1007/s00268-020-05406-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05406-4