Abstract
Background
It is unclear what the exact short-term outcomes of necrotizing soft tissue infections (NSTIs), also known and necrotizing fasciitis of the upper extremity, are and whether these are comparable to other anatomical regions. Therefore, the aim of this study is to assess factors associated with mortality within 30-days and amputation in patients with upper extremity NSTIs.
Methods
A retrospective study over a 20-year time period of all patients treated for NSTIs of the upper extremity was carried out. The primary outcomes were the 30-day mortality rate and the amputation rate in patients admitted to the hospital for upper extremity NSTIs.
Results
Within 20 years, 122 patients with NSTIs of the upper extremity were identified. Thirteen patients (11%) died and 17 patients (14%) underwent amputation. Independent risk factors for mortality were an American Society of Anesthesiologists (ASA) classification of 3 or higher (OR 9.26, 95% CI 1.64–52.31) and a base deficit of 3 meq/L or greater (OR 10.53, 95% CI 1.14–96.98). The independent risk factor for amputation was a NSTI of the non-dominant arm (OR 3.78, 95% CI 1.07–13.35). Length of hospital stay was 15 (IQR 9–21) days.
Conclusion
Upper extremity NSTIs have a relatively low mortality rate, but a relatively high amputation rate compared to studies assessing NSTIs of all anatomical regions. ASA classification and base deficit at admission predict the prognosis of patients with upper extremity NSTIs, while a NSTI of the non-dominant side is a risk factor for limb loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Necrotizing soft tissue infections (NSTIs) are rapidly spreading and progressive infection of the soft tissues, most often affecting the fascia and subcutaneous layers [1,2,3].
The extremities have been described to be affected in 45–74% of all NSTI cases, of which the lower extremity are most commonly involved [2, 4, 5]. NSTIs of the upper extremity have reported frequencies varying between 7 and 27% [6,7,8]. Most of the studies assessing upper extremity NSTIs are limited to small case series or big national database studies with less detail about the presentation and admission itself [6,7,8]. Due to this low representation of upper extremity NSTIs in the literature, it is unknown what the exact short-term outcomes are and whether they are comparable to NSTIs affecting other anatomical regions. Knowledge of risk factors for mortality and morbidity provides insight into the prognosis and possibilities to improve outcomes of NSTIs of the upper extremity.
Therefore, this study aims to assess which factors are associated with mortality within 30 days and amputation in patients with necrotizing soft tissue infections of the upper extremity.
Methods
This study was approved by the hospitals’ institutional review board. A retrospective study of patients treated for NSTIs of the upper extremity at two academic referral centers between January 1998 and January 2018 was carried out. We identified eligible patients from the institution’s Research Patient Data Registry (RPDR) by using the International Classification of Diseases (ICD)-9 (928.86) and ICD-10 (M72.6) codes for necrotizing fasciitis. The search resulted in 1507 patients. All patients diagnosed with NSTIs of the upper extremity were eligible for inclusion and determined the sample size. Since no pathognomonic clinical symptoms are known for NSTIs, the diagnosis needed to be established either by histopathology or by microbiology (e.g., gram stain and/or definitive culture) [1, 9, 10]. Cases in which the disease started at another anatomical region but progressed to one or both upper extremities were included as well. Exclusion criteria were patients <18 years and pregnancy at time of the infection.
Outcome measures and explanatory variables
The primary goals of this study were to describe the 30-day mortality rate and the amputation rate in patients with upper extremity NSTIs. In addition, we described the microbiology, types of procedures performed and hospital course.
The patient demographics extracted from medical charts include age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, comorbidities, medical history, smoking status, history of intravenous drug use and work status. The extracted disease-related characteristics include time from symptoms to diagnosis, affected side, dominant hand, causative event for the NSTI, date of causative event, location where symptoms started, affected body areas, parts of upper extremity affected by the NSTI, vital signs and laboratory results at presentation and causative microorganism found. The extracted treatment-related variables include the hospital of first debridement, amputation, mortality, date of death, intensive care unit (ICU) admittance, length of ICU and hospital stay, type and number of surgeries performed for the NSTI, date of last surgery for the NSTI, infectious complications during admission and discharge location. If the ASA classification was unknown, the researchers determined the ASA classification based on comorbidities known prior to the NSTI. We defined manual laborers as workers mainly doing physical work dominated by grasping and lifting [11]. The extent of the NSTI was approximated by calculating the percentage of total body surface area (TBSA) affected using the rule of nines commonly used in burns [12]. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was calculated if the necessary laboratory results were available. The LRINEC score is used to predict the likelihood of NSTIs. A LRINEC score <6 represents a low suspicion for NSTI [13]. By using the definitive culture results, we classified the NSTIs in type I (polymicrobial), type II (monomicrobial) or type III (e.g., Clostridium spp., Vibrio spp. or gram-negative bacteria) [14].
Statistical analysis
Continuous parametric variables are presented as means with standard deviations (SDs), continuous nonparametric variables as medians with interquartile ranges (IQRs) and categorical variables as frequencies with percentages. Missing data were handled using pairwise deletion. Univariable logistic regressions were used to identify predictors for mortality and amputation. Variables with a p value <0.10 were eligible for inclusion in the multivariable logistic regression with simultaneous entry followed by backward selection. Only the most clinically relevant variables were selected to prevent overfitting the model, and variables with small numbers of occurrence in our cohort (e.g., 6 patients with a specific variable) were not included due to their limited statistical power. For analyses related to surgical procedures, the Mann–Whitney U test was used. For all analyses, a p value of <0.05 was considered statistically significant. Analyses were performed with STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
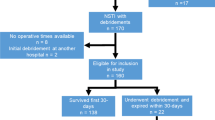
After exclusion, 122 eligible patients were identified with a mean age of 50 ± 17 years (Fig. 1). Patients were predominately classified ASA classification 1 or 2 (n = 74, 62%), which is indicative for no or minor comorbidities. The forearm was involved in most patients (n = 89, 74%). If a causative event was known, an injection was most frequently described (n = 26, 35%), of which 21 cases were caused by self-administrated intravenous drugs (Tables 1, 2).
Mortality within 30 days
Thirteen patients (11%) died within 30 days after the NSTI diagnosis. Nine of these patients (83%) were classified as ASA 3 or 4, indicating severe and/or multiple comorbidities. Patients with fatal NSTIs died at a median of 3 days after hospital admission (IQR 1–8), with the most common cause of death being sepsis (n = 10, 77%) (Table 3). In three other patients (23%), further surgical treatment was averted due to patients’ wishes combined with severe preexisting comorbidities.
Univariable analyses showed that higher age (OR 1.07, 95% CI 1.03–1.12), ASA classification 3 or 4 compared to ASA 1 or 2 (OR 9.00, 95% CI 1.85–43.85), history of intravenous drug use (OR 4.04, 95% CI 1.07–15.50), a higher respiratory rate (OR 1.10, 95% CI 1.00–1.21), a higher glucose (OR 1.00, 95% CI 1.00–1.02), a higher base deficit (OR 1.40, 95% CI 1.14–1.72) or a higher serum lactate at time of diagnosis (OR 1.65, 95% CI 1.17–2.32), NSTIs that started at another anatomical region than the upper extremity (OR 10.60, 95% BI 1.89–21.76), NSTIs at other anatomical regions (especially the trunk and lower extremity) besides the upper extremity (OR 6.33, 95% CI 1.84–21.76), NSTIs affecting a greater TBSA (OR 1.05, 95% CI 1.00–1.10) and NSTIs of the shoulder (OR 3.87, 95% CI 1.10–13.61) resulted in a significantly greater risk at dying of the NSTI (Tables 1, 2, 3).
Multivariable analysis showed that an ASA classification of 3 or higher (OR 9.26, 95% CI 1.64–52.31) and a base deficit of 3 meq/L or greater (OR 10.53, 95% CI 1.14–96.98) are both independent risk factors for mortality (Table 4).
Amputation
Seventeen patients (14%) underwent amputation in an attempt to gain control of the infection, which was either an amputation during the index surgery (n = 9) or performed secondarily (n = 8). Three patients underwent amputation but died eventually. Types of amputation were an amputation of digit(s) (n = 7, 41%), transradial (n = 2, 12%), transhumeral (n = 6, 35%) or forequarter amputation (n = 2, 12%).
Univariable analyses assessing the risk at amputation showed that only NSTIs of the non-dominant side (OR 3.79, 95% CI 1.09–13.16), a higher diastolic blood pressure at presentation (OR 1.05, 95% CI 1.00–1.09) and NSTIs of the hand (OR 5.04; 95% CI 1.37–18.58) had a greater risk at undergoing amputation (Tables 1, 2, 3).
Multivariable analysis showed that NSTIs of the non-dominant side (OR 3.78, 95% CI 1.07–13.35) were an independent risk factor for amputation (Table 4).
Microbiology
NSTIs of the upper extremity were predominately classified as type II (n = 75, n = 70%), of which a Group A beta-hemolytic Streptococcus (GAS) was most commonly isolated (n = 48, 64%). Of these patients, 10 patients (21%) received intravenous immunoglobulins (IVIG). Administration of IVIG was not associated with reduced mortality or amputation rates. Furthermore, 22 patients (20%) had type I NSTIs and 11 patients (10%) had type III NSTIs. Subgroup analysis of the specific isolated microorganisms found no relation between the isolated microorganisms and 30-day mortality or risk at amputation (Table 2 and Appendix 1).
Hospital course
Eighty patients (66%) first presented to an outside hospital; 63 of these patients (79%) were transferred to one of the two academic centers before their first debridement was performed. Transferring patients before the initial debridement did not result in higher mortality or amputation rates (OR 0.38, 95% CI 0.11–1.30 and OR 1.40, 95% CI 0.50–3.96, respectively) (Table 2).
Median length of hospital stay was 15 days (IQR 9–21). Eighty-six patients were admitted to ICU (70%) for a median stay of 3 days (IQR 2–9). During the hospital stay, 71 patients (58%) developed infectious complications (e.g., toxic shock syndrome, sepsis, pneumonia). After hospital discharge, patients were discharged home (n = 63, 68%), to a rehabilitation facility (n = 39, 36%) or transferred to another hospital for further care (n = 1, 1%) (Table 3).
Patients underwent a median of four operations (IQR 3–6, range 0–22). A median of three debridement procedures (IQR 2–4, range 0–10) and a median of one reconstructive procedure (IQR 1–2, range 0–9) were performed. The first attempt at wound closure was made a median of 8 days (IQR 4–12) after diagnosis. Closure of debridement wounds in surviving patients without amputations was done by using skin grafts (n = 50, 54%), delayed primary closure with sutures (n = 25, 27%), flap surgery (n = 9, 10%) and flap surgery combined with skin grafts (n = 8, 9%). There was no difference in number of surgeries between patients requiring amputation for infection control and those with salvaged upper extremities (Appendix 2).
Discussion
The 30-day mortality rate of 11% found in this study is low compared to mortality rates previously described for NSTIs [2, 3, 15, 16]. Over 20 years ago, a cumulative mortality rate of 34% was described [17]. Looking at studies for the past ten years, the mortality rate of NSTIs varies between 6% and 33% [2, 3, 15, 16]. The mortality rate probably decreased due to improvement in awareness and treatment, both in surgical care and in critical care support [9]. The cumulative mortality rate described for upper extremity NSTIs is 18.3% (90 patients died out of 493 included patients in 8 studies), with rates as low as 9% and as high as 36% [6, 18,19,20,21,22,23,24]. The low mortality rate in this study might be caused by the relatively large group of intravenous drug user and type II NSTIs. Both of these groups are known to consist of young patients with few comorbidities, which are two factors previously associated with lower mortality rates [25, 26]. The mortality rate for NSTIs of the extremity has been suggested to be lower compared to NSTIs of other anatomical regions (e.g., head, neck, trunk), since NSTIs at these location are commonly more widespread upon presentation and tend to be more difficult to treat [27].
Only two previous studies have looked at factors associated with mortality in upper extremity NSTIs. Both studies found that patients with preexisting comorbidities and patients presenting with septic symptoms had a greater risk of dying [20, 22]. In this study, the ASA classification was used to assess the overall physical status of the patients and the severity of the comorbidities combined. A higher ASA classification has been linked to higher mortality after major trauma and a wide variety of surgical procedures [28, 29]. Patients with upper extremity NSTIs and major or multiple comorbidities (ASA 3 or 4) had a nine times greater risk of dying compared to patients with no to minimal comorbidities (ASA 1 or 2). This association has not yet been described for upper extremity NSTIs, but has been described by two studies assessing NSTIs of all body regions [2, 4, 30].
Base deficit at admission was also found to predict mortality due to upper extremity NSTIs. Patients with a base deficit ≥3 meq/L at admission had an 11-time greater risk at dying compared to patients with a base deficit of <3 meq/L. A base deficit provides a fast estimate of the physiological disturbance of the patient, is a marker for shock and is usually part of the standard diagnostic armamentarium in the emergency department [31, 32]. Base deficits are already used to predict complications and mortality in trauma and intensive care patients and has been described by Elliot et al. to also predict mortality in NSTI patients [31,32,33,34]. It has been suggested that the LRINEC score might also be predictive for mortality, since this score also looks at markers for sepsis severity in NSTI patients; however, in this study the LRINEC score was not predictive for mortality and was in most patients far below the cutoff point for NSTI suspicion [13, 35].
Amputation was in 14% of the cases required for management of the infection. Previously described amputation rates for NSTIs range from 6% to 28%, while the amputation rate of studies assessing the upper extremity varies between 6% and 36% with a cumulative amputation rate of 9% [19, 21,22,23, 34, 36,37,38]. This rate is lower than seen in our study, which might be related to the lower mortality rate. More patients survived, but the patients who survived might have required more extreme measures, such as amputation, to survive. Only diabetes mellitus and sepsis have previously been associated with amputation in patients with NSTIs of the upper extremity [22]. This study did not find a relation between those factors but did find that patients with NSTIs of the non-dominant arm were more likely to undergo amputation. This seemed not to be influenced by a more distal location of the infection or by an injection, most commonly done in the non-dominant arm, as causative event. Therefore, we hypothesize that this might represent surgical bias. Surgeons might possibly be more likely to resort to amputation if the non-dominant arm was affected, weighing infection control and the remaining functionality of the dominant arm. Fortunately, due to improved awareness and diagnostics, the amputation rate has declined during the last 5 years of the study.
This study is limited by its retrospective nature. First, during the inclusion process patients might have been missed for inclusion due to miscoding of ICD codes or underdiagnosing of NSTIs in deceased patients. Second, the accuracy of retrospective data is determined by the accuracy of reporting of findings in patients’ chart. This may have led to non-differential misclassification or absent variables. This was the case for the exact time (in hours) to diagnosis and surgery. Third, we included patients who first presented to outside hospitals, which results in less detailed information about findings at the initial presentation. Fourth, this study assesses a broad study period during which the management of critical ill patients changed as well. Our data are representative of actual practice including the management variations; however, these variations should be kept in mind. Finally, due to the rarity of NSTIs and the associated small sample sizes and limited occurrence of the outcomes, sparse data bias should be kept in mind during the interpretation of the results. However, the strength of this study is that this is one of the biggest and most detailed studies of NSTIs of the upper extremity.
Conclusion
NSTIs of the upper extremity have a relatively low mortality rate, but a relatively high amputation rate compared to studies assessing NSTIs of all anatomical regions. ASA classification and base deficit at admission can predict the prognosis of patients with upper extremity NSTIs, while a NSTI of the non-dominant arm is a risk factor for limb loss.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ICD:
-
International classification of diseases
- ICU:
-
Intensive care unit
- IVIG:
-
Intravenous immunoglobulins
- IQR:
-
Interquartile range
- GAS:
-
Group A beta-hemolytic streptococcus
- LRINEC:
-
Laboratory Risk Indicator for Necrotizing Fasciitis
- NSTI:
-
Necrotizing soft tissue infection
- OR:
-
Odds ratio
- RPDR:
-
Research Patient Data Registry
- TBSA:
-
Total body surface area
- SD:
-
Standard deviation
References
Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO (2003) Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Jt Surg 85(8):1454–1460
Krieg A, Dizdar L, Verde PE, Knoefel WT (2014) Predictors of mortality for necrotizing soft-tissue infections: a retrospective analysis of 64 cases. Langenbeck’s Arch Surg 399(3):333–341
Audureau E, Hua C, de Prost N, Hemery F, Decousser JW, Bosc R, Lepeule R, Chosidow O, Sbidian E (2017) Mortality of necrotizing fasciitis: relative influence of individual and hospital-level factors, a nationwide multilevel study, France, 2007–12. Br J Dermatol 177(6):1575–1582
Nawijn F, Wassenaar ECE, Smeeing DPJ, Vlaminckx BJM, Reinders JSK, Wille J, Leenen LPH, Hietbrink F (2019) Exhaustion of the immune system by Group A streptococcus necrotizing fasciitis : the occurrence of late secondary infections in a retrospective study. Trauma Surg Acute Care Open 4:e000272
Wang JM, Lim HK (2014) Necrotizing fasciitis: eight-year experience and literature review. Brazilian J Infect Dis 18(2):137–143
Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Yodluangfun S, Tantraworasin A (2015) Necrotizing fasciitis: risk factors of mortality. Risk Manag Healthc Policy 8:1–7
Liu Y, Ho M, Chen C, Liao W, Ho C, Lin P, Wang J (2005) Microbiology and factors affecting mortality in necrotizing fasciitis. Yearb Surg 38:430–435
Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV (2007) Necrotising fasciitis of upper and lower limb: a systematic review. Injury 38(Suppl 5):18–25
Hietbrink F, Bode LG, Riddez L, Leenen LPH, van Dijk MR (2016) Triple diagnostics for early detection of ambivalent necrotizing fasciitis. World J Emerg Surg 11(1):1–7
Anaya DA, Bulger EM, Kwon YS, Kao LS, Evans H, Nathens AB (2009) Predicting death in necrotizing soft tissue infections: a clinical score. Surg Infect (Larchmt) 10(6):517–522
United States Goverment. 41 CFR 61-250.2 - What definitions apply to this part? [Internet], Code of Federal Regulations, 2005 [cited 2018 Dec 1]. https://www.gpo.gov/fdsys/search/pagedetails.action?collectionCode=CFR&browsePath=Title+41%2FSubtitle+B%2FChapter+61%2FPart+61%2FSection+61-250.2&granuleId=CFR-2005-title41-vol1-sec61-250-2&packageId=CFR-2005-title41-vol1&collapse=true&fromBrowse=true
Livingston EH, Lee S (2000) Percentage of burned body surface area determination in obese and nonobese patients. J Surg Res 91(2):106–110
Wong CH, Khin LW, Heng KS, Tan KC, Low CO (2004) The LRINEC (Laboratory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 32(7):1535–1541
Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A (2014) Current concepts in the management of necrotizing fasciitis. Front Surg 1:36
McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA (1995) Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 221(5):558–565
Kao LS, Lew DF, Arab SN, Todd SR, Awad SS, Carrick MM, Corneille MG, Lally KP (2011) Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: a multicenter study. Am J Surg 202(2):139–145
McHenry CR, Brandt CP, Piotrowski JJ, Jacobs DG, Malangoni MA (1994) Idiopathic necrotizing fasciitis: recognition, incidence, and outcome of therapy. Am Surg 60(7):490–494
Schecter W, Meyer A, Schecter G, Giuliano A, Newmeyer W, Kilgore E (1982) Necrotizing fasciitis of the upper extremity. J Hand Surg Am 7(1):15–20
Tang WM, Ho PL, Fung KK, Yuen KY, Leong JCY (2001) Necrotising fasciitis of a limb. J Bone Jt Surg 83(5):709–714
Cheng NC, Su YM, Kuo YS, Tai HC, Tang YB (2008) Factors affecting the mortality of necrotizing fasciitis involving the upper extremities. Surg Today 38(12):1108–1113
Espandar R, Sibdari SY, Rafiee E, Yazdanian S (2011) Necrotizing fasciitis of the extremities: a prospective study. Strateg Trauma Limb Reconstr 6(3):121–125
Uehara K, Yasunaga H, Morizaki Y, Horiguchi H, Fushimi K, Tanaka S (2014) Necrotising soft-tissue infections of the upper limb: risk factors for amputation and death. Bone Jt J 96B(11):1530–1534
Corona PS, Erimeiku F, Reverté-Vinaixa MM, Soldado F, Amat C, Carrera L (2016) Necrotising fasciitis of the extremities: implementation of new management technologies. Injury 47:S66–S71
Lauerman MH, Scalea TM, Eglseder WA, Pensy R, Stein DM, Henry S (2018) Physiology, not modern operative approach, predicts mortality in extremity necrotizing soft tissue infections at a high-volume center. Surgery (United States) 164(1):105–109
Dworkin MS, Westercamp MD, Park L, McIntyre A (2009) The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiol Infect 137(11):1609–1614
Hakkarainen TW, Kopari NM, Pham TN, Evans HL (2014) Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg 51(8):344–362
Trent JT, Kirsner RS (2002) Diagnosing necrotizing fasciitis. Adv Skin Wound Care 15(3):135–138
Skaga NO, Eken T, Søvik S, Jones JM, Steen PA (2007) Pre-injury ASA physical status classification is an independent predictor of mortality after trauma. J Trauma Inj Infect Crit Care 63(5):972–978
Hackett NJ, De Oliveira GS, Jain UK, Kim JYS (2015) ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 18:184–190
Nawijn F, Houwert RM, van Wessem KPJ, Simmermacher RKJ, Govaert GAM, van Dijk MR, de Jong MB, de Bruin IGJ, Leenen LPH, Hietbrink F (2019) A 5-year evaluation of the implementation of triple diagnostics for early detection of severe necrotizing soft tissue disease: a single-center cohort study. World J Surg 43(8):1898–1905. https://doi.org/10.1007/s00268-019-04999-9
Kroezen F, Bijlsma TS, Liem MSL, Meeuwis JD, Leenen LPH (2007) Base deficit-based predictive modeling of outcome in trauma patients admitted to intensive care units in Dutch trauma centers. J Trauma Inj Infect Crit Care 63(4):908–913
Wijaya R, Ng JH, Ong L, Wong ASY (2016) Can venous base excess replace arterial base excess as a marker of early shock and a predictor of survival in trauma? Singap Med J 57(2):73–76
Surbatovic M, Radakovic S, Jevtic M, Filipovic N, Romic P, Popovic N, Jevdjic J, Grujic K, Djordjevic D (2009) Predictive value of serum bicarbonate, arterial base deficit/excess and SAPS III score in critically ill patients. Gen Physiol Biophys 28:271–276
Elliott D, Kufera J, Myers R (1996) Necrotizing soft tissue infections. Ann Surg 224(5):672–683
Colak E, Ozlem N, Kucuk GO, Aktimur R, Kesmer S (2014) Laboratory risk indicators for necrotizing fasciitis and associations with mortality. Turk J Emerg Med 14(1):15–19
Ogilvie CM, Miclau T (2006) Necrotizing soft tissue infections of the extremities and back. Clin Orthop Relat Res 447:179–186
Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A (2015) Necrotizing fasciitis: epidemiology and clinical predictors for amputation. Int J Gen Med 8:195–202
Gonzalez M, Kay T, Weinzweig N, Brown A, Pulvirenti J (1966) Necrotizing fasciitis of the upper extremity. J Hand Surg Am 21A:689–692
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Eberlin is a consultant for AxoGen and Integra. Dr. Chen received grants by Miami Device Solutions, OMeGA and Acumed. All authors declare to have no commercial relations that might pose a conflict of interest in connection with the manuscript.
Ethical approval
This study was approved by the hospitals’ institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nawijn, F., Verhiel, S.H., Lunn, K.N. et al. Factors Associated with Mortality and Amputation Caused by Necrotizing Soft Tissue Infections of the Upper Extremity: A Retrospective Cohort Study. World J Surg 44, 730–740 (2020). https://doi.org/10.1007/s00268-019-05256-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05256-9