Abstract
Background
Parathyroidectomy (PTx) is the treatment of choice for end-stage renal disease (ESRD) patients with therapy-resistant hyperparathyroidism (HPT). The optimal timing of PTx for ESRD-related HPT—before or after kidney transplantation (KTx)—is subject of debate.
Methods
Patients with ESRD-related HPT who underwent both PTx and KTx between 1994 and 2015 were included in a multicenter retrospective study in four university hospitals. Two groups were formed according to treatment sequence: PTx before KTx (PTxKTx) and PTx after KTx (KTxPTx). Primary endpoint was renal function (eGFR, CKD-EPI) between both groups at several time points post-transplantation. Correlation between the timing of PTx and KTx and the course of eGFR was assessed using generalized estimating equations (GEE).
Results
The PTxKTx group consisted of 102 (55.1%) and the KTxPTx group of 83 (44.9%) patients. Recipient age, donor type, PTx type, and pre-KTx PTH levels were significantly different between groups. At 5 years after transplantation, eGFR was similar in the PTxKTx group (eGFR 44.5 ± 4.0 ml/min/1.73 m2) and KTxPTx group (40.0 ± 6.4 ml/min/1.73 m2, p = 0.43). The unadjusted GEE model showed that timing of PTx was not correlated with graft function over time (mean difference −1.0 ml/min/1.73 m2, 95% confidence interval −8.4 to 6.4, p = 0.79). Adjustment for potential confounders including recipient age and sex, various donor characteristics, PTx type, and PTH levels did not materially influence the results.
Conclusions
In this multicenter cohort study, timing of PTx before or after KTx does not independently impact graft function over time.
Similar content being viewed by others
Introduction
Hyperparathyroidism (HPT) is a common metabolic complication in end-stage renal disease (ESRD) [1]. Both before and after kidney transplantation (KTx), HPT has been associated with adverse patient outcomes [2, 3]. Parathyroidectomy (PTx) is the treatment of choice for patients with HPT refractory to pharmacological treatment [4]. Multiple studies have shown that PTx is very effective in lowering PTH levels in ESRD patients, and successful PTx may reduce the risk of all-cause and cardiovascular mortality in ESRD patients with severe uncontrolled HPT [5, 6]. Since secondary HPT may regress in up to 57% of patients with correction of mineral homeostasis after successful KTx, PTx is often postponed in patients listed for transplantation [7,8,9]. On the other hand, persistently elevated parathyroid hormone (PTH) levels cause resistance of the parathyroid to serum calcium levels due to reduced expression of the calcium-sensing receptor [10, 11]. This results in tertiary HPT: autonomous production of PTH with subsequent hypercalcemia, which occurs in 25–50% of patients after KTx [12, 13]. PTx is considered the only definitive treatment for tertiary HPT [14]. The introduction of calcimimetics in 2004 has reduced PTx rates and contributed to prolonged exposure to elevated serum PTH levels [15]. Previous studies suggest that a higher pre-KTx PTH level is associated with accelerated estimated glomerular filtration rate (eGFR) decline after KTx, and an increased risk of graft failure [3, 16, 17]. Furthermore, previous studies suggested a transient deterioration of renal graft function after PTx [16, 18, 19].
These previous studies report discordant results and arguments regarding the optimal timing of PTx in relation to KTx. In this study, we aimed to assess the impact of the sequence of KTx and PTx on the course of post-KTx renal function in a large multicenter cohort of ESRD-related HPT patients with long-term follow-up.
Materials and methods
Study design
We performed a multicenter retrospective cohort study in patients who underwent both KTx and PTx. The Dutch Hyperparathyroidism Study Group (DHSG) initiated a multicenter retrospective database with data from four academic centers in the Netherlands (University Medical Center Groningen [UMCG], Academic Medical Center Amsterdam [AMC], Erasmus Medical Center Rotterdam [EMC], and Leiden University Medical Center [LUMC]). We evaluated medical records of all patients who underwent both a KTx and a PTx in these centers between 1994 and 2015. All patients were ≥18 years and diagnosed with ESRD-related HPT. Patients were divided into two groups according to treatment sequence: the PTxKTx group, who underwent PTx before KTx, and the KTxPTx group, who underwent PTx after KTx. When patients received more than one kidney transplant, only the first KTx was taken into account.
This study was approved by the local medical ethical committee of all participating centers (METc 2014/077). The study was performed according to the Helsinki Ethical Principles.
Data collection
For all patients, we collected cause of ESRD, pre-transplant dialysis status (preemptive or dialysis), history of diabetes mellitus, donor age and sex, cold and warm ischemia times, number of HLA mismatches, primary non-function (PNF), type of PTx (subtotal PTx or total PTx with autotransplantation), and biochemistry. PNF was defined as an eGFR <10 ml/min/1.73 m2 at 3 months after KTx. Patients who reached ESRD (dialysis or re-transplantation) during follow-up were denoted as having an eGFR of 0 ml/min/1.73 m2 until end of follow-up at 5 years; patients who died during follow-up were censored. The following biochemical measurements were recorded: serum calcium, PTH, albumin, and creatinine prior to KTx and PTx and at 3 months, 6 months, 1 year, 3 years, and 5 years after both KTx and PTx. For patients who underwent KTx after 2014, only 3-year follow-up data are available. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) in ml/min/1.73 m2 [20]. Serum calcium and albumin were measured using routine laboratory techniques. Serum calcium was corrected for albumin levels according to the following formula: adjusted total calcium (mg/dL) = measured calcium (mg/dL) + (0.8 * 4 − [albumin (g/dL)]). Reference range for serum calcium was 2.20–2.60 mmol/L. Different PTH assays were used among the four centers. UMCG: until 2006: Nichols Institute Diagnostics, San Juan Capistrano, CA, USA; since February 2006: Immulite 2500, Siemens Healthcare Diagnostics, Deerfield, IL, USA; and the Cobas 3601 immunology analyzes Roche Diagnostics, Mannheim, Germany. PTH values were recalculated according to the conversion equation provided by the laboratory. Reference values were 1.8–9.6 pmol/L. EMC: Vitros ECi Assay, Ortho-Clinical Diagnostics, Inc., New Jersey, USA. Reference range was 1.4–7.3 pmol/L. AMC: Roche Cobas e602, Roche Diagnostics International Ltd, Rotkreuz, Switzerland, with a reference range of 2.00–7.00 pmol/L. LUMC: Immulite 1000, Siemens Healthcare Diagnostics, Deerfield, IL, USA, with a reference range of 0.7–8.0 pmol/L.
Primary and secondary endpoints
To analyze the impact of the timing of PTx on graft function, the primary endpoint was eGFR at 5 years after transplantation. We also analyzed serum-corrected calcium, and PTH, graft failure and post-PTx complications, including temporary palsy of the recurrent laryngeal nerve (RLN), surgical site problems (SSP, including hematoma and infection), hospital-acquired pneumonia (HAP), intensive care unit (ICU) admission, and temporary hypocalcaemia.
Data analyses and statistics
A power analysis was performed based on results of a previous comparable study [21]. With 80% power and a two-sided α = 0.05, a simple size of n = 120 is required to detect a 20% difference in eGFR post-KTx. Statistical analysis was performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, NY, USA); a P value of <0.05 was considered statistically significant.
Patient characteristics were compared between the two groups (PTxKTx vs. KTxPTx) using Mann–Whitney U test and Pearson’s Chi-square test where appropriate. Continuous variables were reported as mean ± standard error of the mean (SEM) or median with interquartile range (IQR). Categorical variables were expressed as number (n) and percentage (%).
To further study the impact of treatment sequence on graft function over time, we established generalized estimating equations (GEE) models with an exchangeable correlations structure. Based on previous literature, recipient age and sex, donor age and sex, type of donor (living vs. deceased), total number of HLA mismatches, type of PTx (total vs. subtotal), cold ischemia time, and pre-transplant dialysis status (preemptive or dialysis) were defined as potential important confounders prior to analysis and were adjusted for in the multivariable GEE model. Furthermore, baseline variables with P values <0.2 in the univariate GEE analyses were included in multivariable GEE model. Results of the GEE model are displayed as estimates of the effects (B) and 95% confidence interval (CI) with P value. In a sub-analysis, we also evaluated the impact on eGFR in patients who underwent PTx shortly after KTx (<1 year) or longer (≥1 year) after KTx.
Results
Patient characteristics
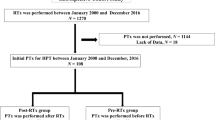
A total of 185 patients were included: 102 (55.1%) patients underwent PTx before KTx (PTxKTx group), while 83 (44.9%) underwent PTx after KTx (KTxPTx group) (Fig. 1). Baseline patient and transplant characteristics are presented in Table 1. Patients within the KTxPTx group were significantly younger at the time of KTx than patients in the PTxKTx group. Eight patients (4.3%) underwent preemptive KTx; these patients were well equally distributed among the groups (n = 3 vs. n = 5, p = 0.31). Median time from start dialysis until KTx was significantly longer in the PTxKTx group compared to the KTxPTx group (61 months [46–83 months] versus 36 months [14–57 months], p <0.01). In the KTxPTx group, living donor KTx was more common compared to the PTxKTx group (26.5% vs. 6.9%, p = 0.006). Pre-KTx PTH levels were significantly higher in the KTxPTx group (66 [34–127] pmol/L vs. 15 [4–35] pmol/L, p <0.001). Calcimimetics were used at some point during follow-up in 31.4% of the patients in the PTxKTx group, compared to 20.5% of the patients in de KTxPTx group (p = 0.18). Ten patients (10.1%) developed primary non-function after KTx in the PTxKTx group, compared to 17.9% in the KTxPTx group (p = 0.13), and were excluded from further analysis.
Patients with PTx before KTx
Patients in the PTxKTx group had a median pre-PTx serum PTH level of 120 (73–186) pmol/L. Postoperatively, PTH dropped with a median of 96 (83–99)% within 3 months. Serum calcium levels corrected for albumin changed after PTx from 10.1 (9.3–10.7) mg/dL to 9.1 (8.1–10.0) mg/dL (p <0.01). Median time from PTx to KTx was 23 (11–38) months (Fig. 2). Median PTH levels increased from 5.4 (2.2–14.7) pmol/L post-PTx to 15 (4–35) pmol/L at day of admission for KTx (p = 0.006). Three months after KTx, PTH levels dropped significantly to 11 (6–24) pmol/L (p = 0.02). The course of eGFR after KTx is presented in Fig. 3. In the PTxKTx group, 8.1% of patients had a complication following PTx (Supplementary Table 1).
Patients with PTx after KTx
Patients in the KTxPTx group underwent PTx at median 30 (15–74) months after KTx (Fig. 2). Fifteen of the 83 patients (18.1%) underwent PTx within 1 year after KTx. Median pre-PTx PTH levels were significantly lower in the KTxPTX group compared to the PTxKTX group (50 [26–122] pmol/L vs. 120 [73–186] pmol/L, p <0.001). The median postoperative PTH drop three months after PTx was 88 (63–96)% to 7.7 [2.7–17.1] pmol/L. Serum calcium levels corrected for albumin also decreased significantly after PTx, from 10.7 (9.8–11.6) mg/dL to 9.2 (8.4–9.9) mg/dL (p <0.01). The eGFR course after KTx is depicted in Fig. 3. eGFR before and after PTx is shown in Fig. 4. Fifteen (18.1%) of patients in the KTxPTx group had a ≥25% decrease of eGFR at 3 months after PTx. (20.7 ± 5.4 ml/min/1.73 m2 vs. 37.9 ± 3.2 ml/min/1.73 m2, p = 0.01). At 1 year after PTx, eGFR was similar to pre-PTx values. There were no significant differences in baseline characteristics between patients with ≥25% decrease in eGFR versus patients with stable eGFR after PTx (Supplementary Table 2). The complication rate in the KTxPTx group was 5.0% and not significantly different compared to the PTxKTx group (p = 0.42).
Impact of PTx timing on post-KTx eGFR
The unadjusted GEE model showed that the timing of PTx was not associated with graft function over time (mean difference −1.0 ml/min/1.73 m2, 95% confidence interval [CI] −8.4 to 6.4, p = 0.79), Table 2). In a model adjusted for donor variables including donor type, donor gender, total number of HLA mismatches, whether the transplantation was preemptive or post-dialysis, donor age, and cold ischemia time, the mean difference in eGFR was −4.8 ml/min/1.73 m2 (96% CI −15.4 to 5.7, p = 0.37). Finally, we constructed a third model adjusted for pre-defined potentially relevant covariates and all baseline variables with a P value of <0.2 in univariate analysis. This analysis also showed that the course of eGFR over time was not significantly different between patients who underwent PTx before KTx or after KTx (Table 2).
Discussion
This large multicenter cohort study showed that in patients with ESRD-related HPT, the timing of parathyroidectomy (PTx) before or after kidney transplantation (KTx) does not independently impact the long-term course of kidney function after KTx.
This finding is in line with a previous single-center study that reported no significant difference in graft function at various time points after KTx between patients who underwent PTx before or after KTx [21]. With 185 patients, our study population is almost twice as large and meets the pre-specified sample size sufficient to detect a 20% difference in eGFR after PTx. Moreover, our follow-up was 5 years compared to 12 months in the previous study. Our results are at variance with two smaller retrospective studies. One study (Schwarz et al. [19]) investigating 76 kidney transplant recipients who underwent PTx showed that nearly half of these patients had an eGFR decline of more than 20%; however, renal function returned to pre-PTx values at 1 year post-PTx, in line with our results. In this study, patients who had this deterioration of graft function had a greater delta PTH decline after PTx compared to those without a deterioration [19]. In the current study, no significant differences were found between the stable eGFR group and the patients who had an eGFR decline ≥25%. This is likely due to the small number of patients with such eGFR decline post-PTx in our cohort. Parikh et al. also reported a significant but transient decline in eGFR post-PTx [16]. Another recent study by Littbarski et al. [22] suggested that, particularly early (<1 year) after transplantation, PTx contributes to renal function loss. Despite the larger sample size in our study (n = 185 vs. 123 patients with long-term follow-up), we could not reproduce this result: neither in patients with early PTx (although only 15 patients underwent PTx in the first year post-transplant in our study), nor in those with later PTx after KTx. Our findings thus challenge the recommendation by Littbarski et al. to conduct PTx before KTx in all patients. The finding of Lou et al. [7] that up to 57% of patients show regression of their HPT within 2 years after a KTx supports the recommendation to wait for 1–2 years after KTx before proceeding with PTx.
We found that pre-PTx PTH levels were significantly lower in the group of patients who underwent PTx after KTx. This could be partly explained by improved renal function resulting in at least partly restored mineral homeostasis [23]. Alternatively, the difference in pre-KTx PTH values could result from the fact that patients with more severe HPT were more likely to undergo PTx prior to receiving a kidney transplant.
Factors determining the post-transplant eGFR course include donor and recipient age and sex, history of diabetes, cold ischemia time, total number of HLA mismatches, and donor type [24]. Our results indicate that the timing of PTx in relation to KTx does not importantly impact the course of renal function. Therefore, other factors than impact on graft function should be taken into account when deciding on a treatment plan and sequence in patients with ESRD-related HPT. These factors include the severity of symptoms, quality of life, patient preference, level of calcium, phosphate and PTH, bone density, and the anticipated chance of spontaneous regression of HPT after KTx [25]. HPT is associated with an increased risk of cardiovascular disease events and mortality [3, 26]. Therefore, when KTx is expected in the near future and the abovementioned factors are taken into account, we suggest to be reticent to proceed with PTx. Based on our results, it seems safe for patients with persistent HPT after KTx to undergo PTx, at least regarding renal function and comparable complication rates between the two groups (Supplementary Table 2). Additionally, there was no significant difference in HPT recurrence rate between both study groups. In a recent study, male sex and white race were predictors of a more pronounced decline in PTH 2 years after KTx, whereas obesity, dialysis vintage, and delayed graft failure were risk factors for persisting HPT after KTx [7]. Studies aimed at identifying predictors of PTH normalization after KTx support a personalized approach toward the timing of PTx in relation to KTx.
Several limitations of our study should be addressed. Inherent to the retrospective nature of our study, selection bias and residual confounding cannot be excluded. Many patient-specific factors have likely driven the decision on PTx timing. We were therefore not able to determine the exact indication criteria for PTx in this multicenter retrospective study cohort. It is, however, probable that patients with more severe disease were more likely to have undergone parathyroidectomy prior to their kidney transplantation compared to the patients with less severe disease. Prospective studies are needed to provide more solid evidence. Second, our results obtained in four centers in the Netherlands might not be extrapolated to other countries with different healthcare infrastructure and guidelines. Third, we could not take the introduction of calcimimetics into account, which has significantly changed management of HPT [15].
In conclusion, we found that the sequence of KTx and PTx does not independently influence post-transplant graft function. We suggest that PTx can be safely performed after KTx in patients with persistent HPT. Therefore, we support the approach to postpone PTx until after KTx if transplantation is expected within a reasonable timeframe in order to allow spontaneous HPT regression, which occurs in a considerable proportion of patients. Our findings support a personalized approach for HPT patients listed for kidney transplantation.
Abbreviations
- PTx:
-
Parathyroidectomy
- ESRD:
-
End-stage renal disease
- HPT:
-
Hyperparathyroidism
- KTx:
-
Kidney transplantation
- eGFR:
-
Estimated glomerular filtration rate
- GEE:
-
Generalized estimating equations
- CI:
-
Confidence interval
- SEM:
-
Standard error of the mean
- PTH:
-
Parathyroid hormone
- DHSG:
-
Dutch Hyperparathyroidism Study Group
- PNF:
-
Primary non-function
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- RLN:
-
Recurrent laryngeal nerve
- SSP:
-
Surgical site problems
- HAP:
-
Hospital-acquired pneumonia
- ICU:
-
Intensive care unit
- PTxKTx:
-
Parathyroidectomy before kidney transplantation
- KTxPTx:
-
Parathyroidectomy after kidney transplantation
- HLA:
-
Human leukocyte antigen
References
Levin A, Bakris GL, Molitch M et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71:31–38
Tentori F, Wang M, Bieber BA et al (2015) Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10:98–109
Pihlstrom H, Dahle DO, Mjoen G et al (2015) Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 99:351–359
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO (2017) Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int 2017:7
Chen J, Zhou QY, Wang JD (2015) Comparison between subtotal parathyroidectomy and total parathyroidectomy with autotransplantation for secondary hyperparathyroidism in patients with chronic renal failure: a meta-analysis. Horm Metab Res 47:643–651
Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M (2015) Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 88:350–359
Lou I, Foley D, Odorico SK et al (2015) How well does renal transplantation cure hyperparathyroidism? Ann Surg 262:653–659
De Boer IH, Gorodetskaya I, Young B, Hsu CY (2002) Chertow GMThe severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13:2762–2769
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y (2004) Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 19:1281–1287
Lewin E, Garfia B, Recio FL, Rodriguez M, Olgaard K (2002) Persistent downregulation of calcium-sensing receptor mRNA in rat parathyroids when severe secondary hyperparathyroidism is reversed by an isogenic kidney transplantation. J Am Soc Nephrol 13:2110–2116
Cozzolino M, Brancaccio D, Gallieni M, Galassi A, Slatopolsky E, Dusso A (2005) Pathogenesis of parathyroid hyperplasia in renal failure. J Nephrol 18:5–8
Lorenz K, Bartsch DK, Sancho JJ, Guigard S, Triponez F (2015) Surgical management of secondary hyperparathyroidism in chronic kidney disease-a consensus report of the European Society of Endocrine Surgeons. Langenbecks Arch Surg. 400:907–927
Bleskestad IH, Bergrem H, Leivestad T, Goransson LG (2011) Intact parathyroid hormone levels in renal transplant patients with normal transplant function. Clin Transplant 25:E566–E570
Lou I, Schneider DF, Leverson G, Foley D, Sippel R, Chen H (2016) Parathyroidectomy is underused in patients with tertiary hyperparathyroidism after renal transplantation. Surgery 159:172–180
van der Plas WY, Engelsman AF, Ozyilmaz A et al (2017) Impact of the introduction of calcimimetics on timing of parathyroidectomy in secondary and tertiary hyperparathyroidism. Ann Surg Oncol 24(1):15–22. https://doi.org/10.1245/s10434-016-5450-6
Parikh S, Nagaraja H, Agarwal A et al (2013) Impact of post-kidney transplant parathyroidectomy on allograft function. Clin Transplant 27:397–402
Callender GG, Malinowski J, Javid M et al (2017) Parathyroidectomy prior to kidney transplant decreases graft failure. Surgery 161:44–50
Evenepoel P, Claes K, Kuypers D, Maes B, Vanrenterghem Y (2005) Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant 20:1714–1720
Schwarz A, Rustien G, Merkel S, Radermacher J, Haller H (2007) HDecreased renal transplant function after parathyroidectomy. Nephrol Dial Transplant 22:584–591
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Jeon HJ, Kim YJ, Kwon HY et al (2012) Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl Int 25:1248–1256
Littbarski SA, Kaltenborn A, Gwiasda J et al (2018) Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathryroidism: effect of pretransplant versus early or late post-transplant parathyroidectomy. Surgery 163:373–380
Baia LC, Heilberg IP, Navis G, de Borst MH (2015) NIGRAM investigatorsPhosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol 11:656–666
Legendre C, Canaud G, Martinez F (2014) Factors influencing long-term outcome after kidney transplantation. Transpl Int 27:19–27
Pitt SC, Sippel RS, Chen H (2009) Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am 89:1227–1239
van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA (2013) Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analysis of prospective studies. Am Heart J 165(655–64):664.e1–664.e5
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local medical ethical committee of all participating centers (METc 2014/077). The study was performed according to the Helsinki Ethical Principles.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Plas, W.Y., El Moumni, M., von Forstner, P.J. et al. Timing of Parathyroidectomy Does Not Influence Renal Function After Kidney Transplantation. World J Surg 43, 1972–1980 (2019). https://doi.org/10.1007/s00268-019-04952-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04952-w