Abstract
Background
There is no consensus on the optimum approach for resection of oesophagogastric junctional (OGJ) tumours. We prospectively evaluated the efficacy of transabdominal radical extended proximal gastrectomy with oesophagogastric anastomosis (EPGOG) for selected tumours of the OGJ.
Methods
Between 1998 and 2007, 66 selected consecutive patients with tumours of the OGJ underwent successful EPGOG. Selection was limited to tumours where the maximal proximal extent was 36 cm ab oral. Pre-, peri-, and postoperative outcomes together with long-term survival data for these patients were prospectively collected.
Results
Median theatre time was 242 min (range = 120–480), with a median blood loss of 300 ml (range = 50–1720). Eighty-nine percent of patients were extubated in theatre; major complications occurred in 9 (14%) patients, with an overall in-hospital mortality rate of 8%. Thirty-five (53%) patients had nodal disease and the median lymph node yield was 13 (range = 4–36), with an R0 resection rate of 80%. In terms of long-term outcomes, the 2- and 5-year actuarial survival rates were 54 ± 6% and 41 ± 6%.
Conclusion
Extended radical proximal gastrectomy with oesophagogastric anastomosis for selected junctional tumours is a feasible technique which does not compromise oncological principles as evidenced by an excellent long-term survival rate.
Similar content being viewed by others
Introduction
Despite advances in the management of oesophageal cancer, patients who undergo surgical resection for oesophagogastric junctional (OGJ) tumours have some of the poorest outcomes of all patients with gastrointestinal tumours [1]. The operations themselves are associated with considerable morbidity and mortality [2], and the long-term survival following resection remains poor [3]. At present there is no consensus on the optimum approach for resection of OGJ tumours. Many centres favour a two-stage Ivor–Lewis procedure, arguing that from an oncological perspective only a combined abdominal and thoracic approach allows adequate lymphadenectomy and locoregional clearance [4]. Others advocate a two-stage abdominocervical approach, with blind transabdominal oesophageal mobilisation in an attempt to avoid the considerable morbidity associated with thoracotomy [5]. A third approach is to perform a transabdominal gastrectomy. Although a number of variations of this single-stage, transabdominal technique have been described in the literature [6, 7], to date there have been no large-scale prospective studies analysing the long-term results of this approach. Over the last decade, our unit has adopted a policy of performing a radical extended proximal gastrectomy with transabdominal, intrathoracic oesophagogastric anastomosis for selected tumours of the OGJ. The purpose of this prospective study was to evaluate the short- and long-term results achieved by this operative approach.
Methods
Our institution is a regional oesophagogastric cancer referral centre. Between April 1998 and August 2007, all patients with histologically confirmed cancer involving the oesophagus who were referred to our centre for consideration for surgery underwent preoperative oncological staging and an assessment of fitness for surgery. Preoperative oncological staging was achieved by a number of techniques, including physical examination, haematological and biochemical investigations, chest X-ray, barium meal, oesophagogastroscopy, abdominal ultrasound, and CT scan. In the latter part of the study, endoscopic ultrasound (from 2004) and positron emission topography (from 2007) were also routinely used to stage the tumours. Fitness for surgery was assessed using a variety of techniques, including clinical examination, pulmonary function tests, electrocardiography, and, where indicated, echocardiography and exercise tolerance testing.
Patients with potentially resectable disease who were deemed physiologically fit for surgery then underwent a diagnostic laparoscopy to exclude occult peritoneal disease. Following this procedure, patients with potentially curative disease proceeded to either surgical resection, or, in the latter part of the study following the publication of the MRC study [8], neoadjuvant chemotherapy as per the MRC protocol followed by surgery. Surgery consisted of either a two-stage transthoracic Ivor–Lewis oesophagectomy or, in selected cases, a transabdominal radical extended proximal gastrectomy with oesophagogastric anastomosis (EPGOG). The selection criteria for the performance of an EPGOG were all tumours of the OGJ with (1) no radiological evidence of mediastinal lymphadenopathy at or above the level of the inferior pulmonary vein, and (2) the maximal proximal extent of the tumour and any associated Barrett’s oesophagus as assessed on endoscopy and (in the latter part of the study) endoscopic ultrasound was 36 cm ab oral and less than 4 cm proximal to the Z line.
During the study period, a total of 182 consecutive patients underwent potentially curative surgery for oesophageal cancer, of whom 67 fulfilled the above criteria and were therefore listed for an EPGOG procedure.
EPGOG technique
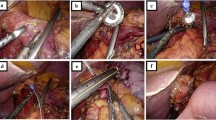
All operations were performed by two surgeons who used a standardised surgical technique. All procedures were performed with the patient under general anaesthesia with thoracic epidural analgesia. An upper midline abdominal incision was used and a multibladed self-retaining system (Omnitract retractor, UA 200, CLS Medical, Newcastle-upon-Tyne, UK) was deployed for cranial and lateral retraction of the thoracic cage. Following exploratory laparotomy to exclude metastatic disease, a Kocher’s manoeuvre was carried out and the stomach was mobilized on the right gastroepiploic vascular pedicle. A spleen-preserving D2-lymphadenectomy (excluding the supra- and infra-pyloric stations 5 and 6) was performed en bloc, and a 5-cm-circumference gastric tube was created from the distal greater curvature of the stomach. Dissection was continued cephalad to include the crura and a cuff of diaphragm which were excised in continuity with the specimen. The flexible narrow blade of the Omnitract retractor was then used to gently retract the heart anteriorly, allowing good access to the mediastinum under direct vision, as described by Alderson et al. [9]. The perioesophageal fat pad was dissected from the pericardium and the medial mediastinal pleurae were excised en bloc. Forward retraction of the stomach also allowed dissection of the posterior perioesophageal tissue from the aorta. The dissection was continued to the level of the inferior pulmonary veins, including the paraoesophageal and para-aortic nodal groups, with preservation of the azygos vein. A 2-0 prolene stay suture was inserted into the oesophagus at the level of the inferior pulmonary vein and the oesophagus was transected, ensuring a macroscopically clear proximal margin of at least 5 cm. Frozen-section histological analysis of the proximal margin was not routinely performed. The anvil of a circular stapler (Premium Plus CEEA stapler, AutoSuture, Ascot, UK) was then introduced into the proximal oesophageal lumen, and the stapler gun was introduced through a 4-cm pylorogastrotomy incision. A stapled oesophagogastrectomy restored intestinal continuity and the pylorotomy was closed as a pyloroplasty. Bilateral transabdominal chest drains and a feeding jejenostomy were inserted, and the abdominal incision was closed to complete the operation.
Immediately following surgery, patients were transferred to the intensive care unit (ICU), or in the latter part of the study, a purpose-built Surgical High Care ward. Assuming satisfactory clinical progress, patients were then transferred to a specialised upper GI ward. Routine gastrograffin swallow contrast studies were performed on postoperative day 5 prior to instituting oral intake. Following discharge from hospital, all patients were regularly followed up in the outpatient clinic. The mean follow-up period was 7.4 ± 0.3 years, with a minimum follow-up period of 1.7 years.
Pathological analysis
The oesophagogastrectomy specimen, together with any separately harvested lymph nodes, was placed in formalin and transported to the laboratory. On arrival, the specimen was opened, avoiding where possible the tumour-bearing portion of the oesophagus. The specimen was serially sectioned in the transverse plane and multiple blocks embedded. The histological subtype, T-stage, and presence of Barrett’s metaplasia were then recorded using the UICC TNM classification [10]. The tumours were further subclassified depending on their anatomical site of origin, as per the Siewert system [11], based on a combination of the results obtained by preoperative endoscopy and histological analysis of the resected specimen. However, in line with previous studies [12–14], all junctional tumours were treated as a single clinical entity and, for reasons of consistency, staged using the TNM criteria for oesophageal cancer. All harvested lymph nodes, together with those retrieved from the oesophagogastrectomy specimen, were then examined for the presence of metastatic tumour deposits. Circumferential resection margin (CRM) involvement was ascertained by examining the sections with maximal lateral spread of tumour. Cases where the distance between the tumour and the serosal margin was less than 1 mm were deemed to have an involved CRM. A positive proximal or distal resection margin was defined as microscopic tumour present in the proximal or distal donut, or tumour present within 1 mm of the longitudinal resection margin of the specimen. An R0 resection was defined as complete tumour excision with all margins histologically free of tumour; an R1 resection as macroscopically complete with microscopically positive proximal, distal, or circumferential margins; and an R2 resection as macroscopically incomplete.
Data analysis
Details of age, gender, operative time (including all anaesthetic procedures), blood loss, length of ICU stay, ventilatory requirements, complications, length of hospital stay, operative mortality (defined as any death occurring during hospitalisation for surgery or within 30 days of the operation if the patient had been discharged), and pathological data were prospective recorded. Data were expressed as mean (±standard error) or median (with ranges in parentheses) as appropriate. Long-term outcome and survival data were collated from medical case notes, General Practitioner records, and Public Health records. Long-term actuarial survival was calculated using the Kaplan-Meier method and expressed as percentages (±standard error) with 95% confidence intervals.
Results
Of the 67 patients listed for EPGOG, this approach was successfully achieved in 66 cases. In one female patient the operation required modification to an Ivor–Lewis resection due to disruption of the oesophageal purse-string and retraction of the proximal end of the oesophagus into the chest prior to anastomosis. The demographic and clinical features of the remaining of 66 patients are summarised in Table 1.
Perioperative outcomes
There were no operative deaths, and postoperatively, 59 patients were extubated in the theatre at the end of surgery and 50 patients were admitted to ICU. This was an overnight stay for 38 of the 50 cases, and only 7 patients required ventilator support once on ICU. The median inpatient postoperative stay was 13 days (range = 8–90 days).
Postoperative complications
A total of 17 patients (26%) suffered immediate postoperative complications, which are listed in Table 2. Of these 17 patients, 9 (14%) had major complications requiring readmission to ICU of whom 5 (8%) died in hospital. The causes of death of these five patients were thoracic aneurysm rupture following conservative management of a small posterior leak; respiratory failure secondary to haemorrhage into the airway during insertion of a tracheostomy; small bowel infarction; and two cases of generalised sepsis secondary to anastomotic leak.
Pathological data
Location of tumours: Of the 66 patients, 20 had a Type I, 22 had a Type II and 24 had a Type III tumour as per the Siewert system [10].
Histological cell type: Of the 66 patients, 4 had no residual tumour in the resected specimen (in three of these cases the patients had undergone previous endoscopic mucosal resections with microscopically involved margins and hence were referred for surgery; in the fourth case, the patient had a complete response to neoadjuvant chemotherapy), 2 patients had adenocarcinoma in situ, 2 patients had squamous cell carcinoma, and 58 had adenocarcinoma.
TNM stage: The final histological T stages were pT0 (4 patients), pTis (2 patients), pT1 (8 patients), pT2 (17 patients), pT3 (30 patients), and pT4 (5 patients). The median lymphadenectomy yield was 13 (range = 4–36), and 35 (53%) patients had metastatic nodal disease.
Resection margins: There were no R2 resections. In terms of R1 resections, ten patients had an involved circumferential resection margin and six patients had microscopic tumour present in the proximal resection margin. Of the latter six patients, three also had an involved circumferential margin and five underwent EPGOG prior to the introduction of routine preoperative staging with endoscopic ultrasound. None of the patients had an involved distal margin meaning that an R0 resection was achieved in 53 (80%) patients.
Long-term outcomes
Following discharge from hospital, none of the patients underwent postoperative chemo- or radiotherapy. A total of 38 patients died following discharge from hospital. One patient died 36 days post surgery from massive GI bleeding; one patient died after revision surgery for hiccoughs and dysphagia 52 months after discharge; and one patient died of unrelated causes 18 months post-EPGOG. The remaining 35 patients died of cancer recurrence (6 of local recurrence alone, the remainder with distant disease). The survival curves for the whole cohort and subdivided according to T stage and N stage are shown in Figs. 1, 2, and 3. The overall actuarial 2- and 5-year survival rates were 54 ± 6% and 41 ± 6%, respectively.
Discussion
Over the last 20 years, there has been an alarming increase in the incidence of adenocarcinoma of the oesophagogastric junction, particularly in Western countries [15]. This increase in disease has fuelled a debate as to the optimum surgical approach for these junctional tumours: Should an extensive transthoracic resection be done to optimise long-term survival? Or should a blind transabdominal oesophageal mobilisation be performed in order to lower perioperative morbidity and mortality rates? Despite a number of studies on this topic, the superiority of one technique over the other has yet to be established [16, 17]. In view of this controversy, we chose to perform radical extended proximal gastrectomy with oesophagogastrostomy (EPGOG) for selected junctional tumours. The rationale for this approach was to maintain oncological principles through en bloc tumour resection and lymphadenectomy under vision, whilst at the same time avoiding the morbidity associated with thoracotomy. It should be noted that this technique was confined to selected patients, specifically those with no radiological evidence of proximal mediastinal lymphadenopathy and where the maximal proximal extent of tumour and any associated Barrett’s oesophagus was 36 cm ab oral. Despite these restrictions, these criteria were fulfilled in over a third of all patients who presented with resectable oesophageal cancer. Moreover, the fact that the transabdominal approach was successfully completed in all but one of these patients demonstrates the technical feasibility of EPGOG.
In terms of the perioperative outcomes of our cohort, EPGOG does appear to have significant advantages over previously published series of transthoracic oesophagectomies. First, our operative time is approximately 1 h shorter than the average reported for transthoracic procedures [16]. In addition, the vast majority of our patients who underwent EPGOG were extubated following surgery, with only 10% requiring mechanical ventilation on ICU. This is in contrast with most series of transthoracic oesophagectomies where patients were typically ventilated post-surgery and spent a considerable period of time on ICU [16]. This has significant resource implications because in many units a transthoracic oesophagectomy cannot be started unless an ICU bed is available postoperatively, a situation that may not necessarily be true for EPGOG.
There are those who champion the technique of transhiatal oesophagectomy with blind transabdominal oesophageal mobilisation and cervical oesophagogastric anastomosis [5]. Undoubtedly, the morbidity and mortality associated with an anastomotic leak in the neck are less than the mediastinitis associated with an intrathoracic leak. However, compared with EPGOG, the need for cervical dissection increases the operating time and the risk of other complications such as chylothorax and recurrent laryngeal nerve damage [16, 17]. In addition, the blind thoracic dissection associated with transhiatal oesophagectomy is associated with intraoperative cardiac embarrassment and a reported incidence of postoperative atrial fibrillation of up to 16% [16]. By contrast, our technique of controlled retraction of the heart using the flexible central blade of the Omnitract [9] appears to lead to better exposure and less cardiac embarrassment as evidenced by our low incidence of postoperative cardiovascular complications. Moreover, our technique of transabdominal, intrathoracic oesophageal dissection under vision is associated with a significantly lower volume of blood loss compared with a previously published series of transhiatal procedures [16]. Overall, we believe that the relatively low rate of morbidity associated with EPGOG suggests that our technique of transabdominal, intrathoracic anastomosis provides at least perioperative outcomes comparable to transhiatal approaches utilising cervical anastomoses.
Although our overall morbidity rate was low, our in-hospital mortality rate of 8% is higher than some recently published series [18]. It should be noted that this study encompasses a historical cohort of patients (many of whom were operated on over a decade ago) and as such, our mortality rate is comparable with that of some contemporaneous series [3] as well as with recently published multicentre results from other UK institutions [19]. Nonetheless, we accept that our mortality rate is disappointingly high and this is an issue we are currently attempting to address. In particular, it has not escaped our notice that our EPGOG technique is amenable to a totally laparoscopic approach [7]. Although during the period of this study we undertook only open surgery, we are currently developing the laparoscopic technique with the aim of reducing the morbidity and mortality associated with EPGOG.
With reference to the pathological outcomes of surgery, our overall R0 resection rate was 80%, which compares very favourably with that of previous studies where rates were between 53 and 80% [20]. Although only 15% of our patients had circumferential resection margin involvement, less encouraging was that 9% of our patients did have an involved positive proximal resection margin. This figure, whilst disappointing, is comparable to those reported by previous large-scale studies [3, 21]. It should also be noted that the majority of our cases with involved proximal margins occurred in the early part of this study, and it is possible that improved preoperative staging with endoscopic ultrasound in the later part of the study may have led to this reduction in the incidence of involved proximal margins over time. Moreover, although during the period of this study we did not perform intraoperative frozen section analysis of the proximal margin, we are now performing this on a semiroutine basis with the aim of reducing our future incidence of R1 resections.
Many proponents of transthoracic oesophagectomy emphasise the superior lymphadenectomy that can be attained using this particular technique [4]. Although it is well established that lymph node status provides the most accurate prognostic information, it is not certain whether an attempt to excise all involved lymph nodes actually improves long-term prognosis [16, 17]. In our series, the median lymph node yield was 13 and 53% of patients had metastatic lymph node involvement. Although this lymph node yield is between a third and a quarter of that reported in previous large-scale series of transthoracic oesophagectomies [4, 16, 17], it is noticeable that the proportion of our patients with involved nodes is comparable to that of these large-scale studies, suggesting that EPGOG did not result in pathological down-staging of tumours. Although some might argue that our relatively low lymph node harvest is a deficiency of our technique [22], it should be noted that our actuarial 5-year survival rate was an impressive 41%. This figure is particularly noteworthy given that the majority of our patients did not receive preoperative chemotherapy as many underwent surgery prior to the publication of the MRC study [6]. This long-term survival rate is superior to those reported by the majority of previous large-scale case series [2, 3, 5, 16, 17, 21, 23] and is comparable to that of a recently published cohort study conducted in the era of routine neoadjuvant chemotherapy [18]. In view of these findings, we would argue that EPGOG provides adequate lymphadenectomy to allow accurate staging, and that the increased morbidity associated with a more extensive transthoracic lymphadenectomy cannot be justified given the already excellent long-term outcomes of our cohort.
We acknowledge that there are some weaknesses in this study. Given the long time period that this study was conducted, there is some heterogeneity with regard to the preoperative management of the cohort due to changes such as the adoption of preoperative chemotherapy following the publication of the MRC study [8]. In addition, we accept that our EPGOG technique does have some shortcomings with regard to our relatively high incidence of postoperative mortality and proximal margin involvement, although with modifications to operative technique as previously described, we are hopeful that both these figures can be reduced in the future. Finally, we did not conduct detailed evaluations of the patients’ quality of life after surgery, and in particular we did not formally assess the incidence of reflux in our cohort. Nonetheless, the prospective nature of this study together with our standardised surgical approach and our long follow-up period adds considerable weight to the validity of our findings.
In summary, we have demonstrated that extended radical proximal gastrectomy for selected OGJ tumours is an oncologically valid technique as demonstrated by our low R0 resection rate and excellent long-term survival outcomes. However, the potential superiority of EPGOG over transthoracic or abdominocervical approaches requires assessment in a larger-scale randomised controlled trial.
References
Muller JM, Ersami H, Stelzner M et al (1990) Surgical therapy of oesophageal cancer. Br J Surg 77:845–857
Ferguson MK, Durkin AE (2002) Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg 123:661–669
Alexiou C, Khan OA, Black E, Field ML, Onyeaka CVP, Beggs L, Duffy JP, Beggs FD (2006) Survival after esophageal resection for carcinoma: the importance of the histological cell type. Ann Thorac Surg 82:1073–1077
Altorki M, Skinner D (2001) Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg 234:581–587
Yannopoulos P, Theodoridis P, Manes K (2009) Esophagectomy without thoracotomy: 25 years of experience over 750 patients. Langenbecks Arch Surg 394:611–616
Wayman J, Dresner SM, Raimes SA, Griffin SM (1999) Transhiatal approach to total gastrectomy for adenocarcinoma of the gastric cardia. Br J Surg 86:536–540
Costi R, Himpens J, Bruyns J, Cadière GB (2004) Totally laparoscopic transhiatal esophago-gastrectomy without thoracic or cervical access. The least invasive surgery for adenocarcinoma of the cardia? Surg Endosc 18:629–632
Medical Research Council Oesophageal Cancer Working Party (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359:1727–1733
Alderson D, Courtney SP, Kennedy RH (1994) Radical transhiatal oesophagectomy under direct vision. Br J Surg 81:404–407
Sobin LH, Wittekind CH (eds) (2002) UICC TNM classification of malignant tumours, 6th edn. Wiley, Hoboken
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459
Wijnhoven BP, Siersema PD, Hop WC, van Dekken H, Tilanus HW (1999) Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity—Rotterdam Oesophageal Tumour Study Group. Br J Surg 86:529–535
Ellis FH Jr, Heatley GJ, Balogh K (1997) Proposal for improved staging criteria for carcinoma of the esophagus and cardia. Eur J Cardiothorac Surg 12:361–364
van de Ven C, De Leyn P, Coosemans W, Van Raemdonck D, Lerut T (1999) Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg 15:769–773
Blot WJ, Devesa SS, Kneller RW, Fraumeni JF (1991) Rising incidence of adenocarcinoma of the oesophagus and gastric cardia. JAMA 265:1287–1289
Hulscher JB, Tijssen JG, Obertop H, van Lanschot JB (2001) Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 72:306–313
Hulscher JB, van Sandick JW, de Boer AG et al (2002) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347:1662–1669
Davies AR, Forshaw MJ, Khan AA, Noorani AS, Patel VM, Strauss DC, Mason RC (2008) Transhiatal esophagectomy in a high volume institution. World J Surg Oncol 6:88
Park DP, Welch CA, Harrison DA et al (2009) Outcomes following oesophagectomy in patients with oesophageal cancer: a secondary analysis of the INARC Case Mix Programme Database. Crit Care 13(S2):S1
Khan OA, Cruttenden-Wood D, Toh SK (2010) Is an involved circumferential resection margin following oesphagectomy for cancer an important prognostic indicator? Interact Cardiovasc Thorac Surg 11:645–648
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067
Rizk NP, Ishwaran H, Rice TW et al (2010) Optimum lymphadenectomy for esophageal cancer. Ann Surg 251:46–50
Khan OA, Fitzgerald JJ, Soomro I, Beggs FD, Morgan WE, Duffy JP (2003) Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer. Br J Cancer 88:1549–1552
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, O., Goh, S., Byrne, B. et al. Long-term Outcomes of Extended Proximal Gastrectomy for Oesophagogastric Junctional Tumours. World J Surg 35, 2245–2251 (2011). https://doi.org/10.1007/s00268-011-1235-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1235-z