Abstract
Background
Eyelid surgery is one of the top five aesthetic procedures. It is performed to improve both appearance and function, but intraoperative bleeding leads to adverse events which perturb patients. The objective of this study was to demonstrate the efficacy of TXA combined with epinephrine in decreasing intraoperative blood loss and postoperative inflammation.
Methods
This prospective randomized control trial was performed on the 30 eyelids of 15 patients who underwent upper blepharoplasty. One of each patient’s eyes was randomly assigned to the TXA group, and the other eye was in the control group. Eyes in the TXA group were given 2% lidocaine with epinephrine (1:100000) mixed with TXA (50 mg/ml) in 1:1 mixture subcutaneously as a local anesthetic. The eyes in the control group received 2% lidocaine with epinephrine (1:100000) diluted with normal saline in 1:1 mixture. Intraoperative blood loss and postoperative swelling were compared between the two groups.
Results
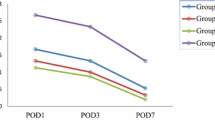
Intraoperative blood loss was significantly higher in the TXA group [4.86 (1.83) ml] than it was in the control group [2.53 (1.49) ml] (p < 0.001). There was no statistically significant difference between the two groups in operative time (p = 0.645), pain score (p = 0.498), lid crease (p = 0.548), or MRD1 (p = 0.626). On postoperative day 7, there was no difference in lid crease (p = 0.879), MRD1 (p = 0.463), pain score (p = 0.934), or ecchymosis (p = 0.976) between two groups.
Conclusions
TXA in lidocaine with epinephrine was found to increase intraoperative bleeding compared to lidocaine with epinephrine alone, but there was no difference in postoperative swelling or ecchymosis. TXA combined with lidocaine and epinephrine injected subcutaneously should be avoided until additional relevant data are obtained. Further drug interaction study is needed.
Level of Evidence II
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper blepharoplasty is performed to treat dermatochalasis and ptosis and in Asian people, for aesthetics [1]. Eyelid surgery is one of the top five aesthetic procedures. Most blepharoplasty operations are performed in an outpatient setting under local anesthesia. But, following the operation, many patients are concerned about swelling and ecchymosis from inflammatory response. Intraoperative bleeding affects postoperative swelling and ecchymosis [2].
The most common anesthetic drug used in upper blepharoplasty is lidocaine with epinephrine. Epinephrine stimulates alpha1 receptors, which constricts peripheral blood vessels and decreases intraoperative bleeding.
Tranexamic acid (TXA) is a lysine analog antifibrinolytic agent. It inhibits fibrin degradation and increases clot stabilization, and it may also attenuate inflammatory response [3]. In previous studies, it reduced inflammatory cytokines and biochemical markers such as IL-6, fibrin separation products, and creatine-kinase [4, 5]. TXA has been used intravenously in cardiothoracic surgery to decrease intraoperative bleeding and minimize intraoperative and postoperative blood transfusion [6]. It has been injected intra-articularly in knee surgeries to reduce bleeding [7]. Topical TXA is used in many operations to reduce blood loss and for moistening and irrigation [8, 9]. In epistaxis patients, local nasal packing soaked in TXA can reduce rebleeding comparably to anterior nasal packing [10]. TXA combined with epinephrine has been used in local wound soaking as anesthetic and to reduce bleeding [11]. TXA has been added to anesthetic drugs for subcutaneous administration in many operations, but the data on its efficacy are limited. The purpose of this study was to demonstrate the efficacy of TXA combined with epinephrine in decreasing intraoperative blood loss and postoperative inflammation.
Patients and Methods
This study was a single center, double blinded, prospective randomized control trial in patients who underwent upper blepharoplasty by a single surgeon at Ramathibodi Hospital, between January 2020 and December 2021. The primary outcome of this study was intraoperative blood loss, and secondary outcomes were operation time, degree of postoperative inflammation assessed via swelling, ecchymosis, and pain on day 7 postoperation. This study was approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University, and was TCTR registered (TCTR20200319009).
Inclusion criteria were patients who underwent upper blepharoplasty, were over 18years old, and provided written informed consent to participate in the study. Exclusion criteria were patients who had ptosis, hypertension (systolic blood pressure [SBP] > 160 mmHg), previous eyelid surgery, history of thromboembolism, seizure, TXA allergy, or were pregnant. Preoperative lid creases (mm) and marginal reflex distance 1 [MRD1] (mm) were measured with a standard caliper (Fig. 1). Photographs were taken of anteroposterior view of full face and standard view of eyes with both eyes open and gently closed.
Patients who were receiving anticoagulants were advised to discontinue taking them at least 7 days before the operation. Hypertensive patients were advised to continue their anti-hypertensive drugs and to keep their SBP under 160 mmHg.
Each patient’s eyes were randomly assigned to either of two groups, but not both to the same group, by a computer program. The first group (TXA group) was given 2% lidocaine with epinephrine (1:100000) mixed with TXA (50 mg/ml) in 1:1 mixture subcutaneously as local anesthetic, and the second group (control group) received 2% lidocaine with epinephrine (1:100000) diluted with normal saline in 1:1 mixture. The anesthetic regimen for each eye was blinded for the surgeon, the researcher, and the patients.
Intraoperative Evaluation
Upper blepharoplasty was performed under a randomized anesthetic regimen. The anesthetic medication was prepared for each eye by an unblinded scrub nurse. The operation always started on the right eye. After the eyelid was prepared using a sterile technique, the assigned anesthetic regimen was injected subcutaneously, and the surgeon waited for 10 min before starting the operation. Intraoperative bleeding was stopped with a standard electrocautery device. Intraoperative blood loss was collected with cottonoids and separated for each eye in zip lock bags to avoid evaporation (Fig. 2). After the operation, the bags with blood-soaked cottonoids were weighed by a blinded researcher, and the net total blood loss in each eye was calculated. Operation time and the amount of anesthetic drug (ml) used for each eye was recorded. Pain during anesthetic drug injection was evaluated by the patients with a visual analog scale from 0 to 10.
Immediate Postoperative Evaluation and Management
With the patient in an upright position, anteroposterior views of full face and standard views of eyes with both eyes open and gently closed were photographed. Lid crease (mm) and MRD1 (mm) were measured. Patients were advised regarding local wound care and cold packing. Prophylaxis antibiotics and paracetamol for pain control were provided.
7-day Postoperative Evaluation
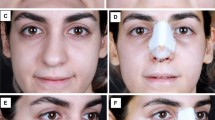
On day 7 postoperation, sutures were removed, and patients were advised regarding scar prevention. Lid crease (mm) and MRD1 (mm) were re-evaluated. With the patient in an upright position, anteroposterior views of full face and standard views of eyes with both eyes open and gently closed were rephotographed. Pain score was evaluated. Ecchymosis was independently reviewed and evaluated from the photographs by the researcher, a plastic surgery resident, and an experienced plastic surgeon who did not perform the surgery. The area of ecchymosis was calculated in percentage compared to the periorbital area on each eye using AutoCAD (Version R.47.M.87, Autodesk, USA) (Fig. 3).
Statistical Analysis
Descriptive statistics were analyzed using mean, percentage, and SD. Independent pair t test was performed to compare between the two groups, with P value < 0.05 indicating significant difference. Statistical analysis was carried out with IBM SPSS Statistics (Version 16.0, USA).
Results
Eighteen patients were enrolled in this study. Three patients were excluded due to ptosis. Finally, fifteen patients with thirty eyelids were included in the study. The patient’s mean (SD) age was 57.6 (6.7) years. Fourteen patients (93%) were female. Preoperative diagnosis was dermatochalasis for all fifteen patients (Table 1).
Intraoperative Results
The results are summarized in Table 2. Intraoperative blood loss was significantly higher in the TXA group [4.86 (1.83) ml] compared with the control group [2.53 (1.49) ml] (p < 0.001). There were no statistically significant differences in volume of anesthetic drug (p = 0.962), operative time (p = 0.645), pain score (p = 0.498), lid crease (p = 0.548) or MRD1 (p = 0.626) between the two groups, but the TXA group tended to have longer operation time [13.17 (7.86) vs. 11.94 (6.61) min] and higher pain score [4.20 (2.40) vs. 3.57 (2.65)] than the control group.
Postoperative Results
Postoperative evaluation was performed on postoperation day 7 (Figs. 4 and 5). There was no significant difference between the two groups in lid crease (p = 0.879), MRD1 (p = 0.463), pain score (p = 0.934), or ecchymosis (calculated as percentage of area, p = 0.976) (Table 2). No patients presented with postoperative complication.
Discussion
TXA is a lysine analog antifibrinolytic agent that inhibits fibrin degradation and increases clot stabilization. Previous studies found that tranexamic acid also decreased inflammatory response. TXA is widely used in many surgical procedures to decrease blood loss during an operation.
A prospective study by Zilinsky et al. [12] showed that subcutaneous injection of TXA reduced bleeding during dermatologic surgery. In contrast, a prospective study by Sagiv et al. [13] showed that subcutaneous TXA reduced intraoperative and postoperative hemorrhaging in upper blepharoplasty only as well as a placebo. Most studies have compared between TXA + epinephrine and TXA alone [14, 15]. There is not much research comparing TXA + epinephrine to TXA + epinephrine + lidocaine in subcutaneous anesthetic.
This prospective randomized control trial is a study of the efficacy of TXA combined with epinephrine to reduce intraoperative bleeding and postoperative inflammation when injected subcutaneously. The efficacy of TXA compared to the control was studied in the same individuals to minimize intrapersonal confounders. Interestingly, intraoperative bleeding was significantly higher in the TXA group compared to the control group. That result contradicted our hypothesis and prior study results. Perhaps, when injected subcutaneously, the acidity of TXA reduced the activity of epinephrine, or TXA molecules might have bound with epinephrine molecules or vascular adrenergic receptors, inhibiting epinephrine activity. Additional research on interaction between TXA and epinephrine is needed. Furthermore, this study did not demonstrate any difference in postoperative swelling and ecchymosis, implying zero efficacy in reducing postoperative inflammation.
A limitation of this study is the small sample size, which was due to the COVID-19 pandemic limiting the number of patients with aesthetic procedures during the study period. Further prospective studies with larger populations should be performed before drawing any conclusions.
Conclusions
TXA in lidocaine with epinephrine was found to increase intraoperative bleeding compared to lidocaine with epinephrine alone, while there was no difference in postoperative swelling and ecchymosis. TXA combined with lidocaine and epinephrine injected subcutaneously should be avoided until additional relevant data are obtained. Further studies on drug interaction between TXA and epinephrine and larger clinical trials are needed.
References
Julius F Jr, Ellis M (2018) Blepharoplasty. In: Rubin JP (ed) Plastic surgery, vol 2. Elsevier, New York, p 33
Lelli GJ Jr, Lisman RD (2010) Blepharoplasty complications. Plast Reconstr Surg 125(3):1007–1017
McCormack PL (2012) Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 72(5):585–617
Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I et al (2007) Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care 11:R117
Jiménez JJ, Iribarren JL, Brouard M, Hernández D, Palmero S, Jiménez A et al (2011) Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dose-dependent, phase IV clinical trial. J Cardiothorac Surg 6(1):138
Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T et al (2017) Tranexamic acid in patients undergoing coronary-artery surgery. New Engl J Med 376(2):136–148
Meena S, Benazzo F, Dwivedi S, Ghiara M (2017) Topical versus intravenous tranexamic acid in total knee arthroplasty. J Orthop Surg 25(1):2309499016684300
Ausen K, Fossmark R, Spigset O, Pleym H (2015) Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br J Surg 102(11):1348–1353
Eftekharian H, Vahedi R, Karagah T, Tabrizi R (2015) Effect of tranexamic acid irrigation on perioperative blood loss during orthognathic surgery: a double-blind, randomized controlled clinical trial. J Oral Maxillofac Surg 73(1):129–133
Zahed R, MousaviJazayeri MH, Naderi A, Naderpour Z, Saeedi M (2018) Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs: randomized controlled trial. Acad Emerg Med 25(3):261–266
Zilinsky I, Barazani TB, Shenkman B, Weisman O, Farber N, Martinowitz U (2016) Topical hemostatic–anesthetic solution to reduce bleeding during mohs micrographic surgery: a case control study. J Drugs Dermatol 15(7):851–855
Zilinsky I, Barazani TB, Visentin D, Ahuja K, Martinowitz U, Haik J (2019) Subcutaneous injection of tranexamic acid to reduce bleeding during dermatologic surgery: a double-blind, placebo-controlled. Randomized Clin Trial. Dermatol Surg. 45(6):759–767
Sagiv O, Rosenfeld E, Kalderon E et al (2018) Subcutaneous tranexamic acid in upper eyelid blepharoplasty: a prospective randomized pilot study. Can J Ophthalmol 53(6):600–604
Wu YG, Zeng Y, Hu QS, Bao XC, Xiong HZ, Shen B (2018) Tranexamic acid plus low-dose epinephrine reduces blood loss in total knee arthroplasty: a systematic review and meta-analysis. Orthop Surg 10(4):287–295
Liu JL, Zeng WN, Wang FY et al (2018) Effects of low-dose epinephrine on perioperative hemostasis and inflammatory reaction in major surgical operations: a randomized clinical trial. J Thromb Haemost 16(1):74–82
Funding
Open access funding provided by Mahidol University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
This study was approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University.
Consent to participate
This study has been obtained informed consent from each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaichumporn, T., Kanokkangsadal, P. & Sarovath, A. Tranexamic Acid Subcutaneously Administered with Epinephrine and Lidocaine in Upper Blepharoplasty: A Randomized Double-Blind Control Trial. Aesth Plast Surg (2024). https://doi.org/10.1007/s00266-024-04112-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00266-024-04112-z