Abstract
Background
The goals of mastopexy differ significantly from those of augmentation mammoplasty. Mastopexy is designed to lift and reshape the breasts, while augmentation mammoplasty is designed to increase the volume of the breasts. This conflict causes that one-stage augmentation mastopexies showed a revision rate from 8.7 to 23.2%. The aim of our study is to present some technical refinements for reducing the risk of implant exposure and reoperation.
Methods
We designed a retrospective matched cohort study, including 216 consecutive patients, undergone augmentation mastopexy between January 2013 and December 2022. We divided them in two groups: Group A undergone an inverted-T superomedial pedicled augmentation mastopexy and Group B undergone our inverted-T modified augmentation mastopexy. The groups were matched for clinical and surgical variables, with the surgical technique the only difference between the two.
Results
Complications were registered in ten patients (9.3%) in Group A (two wound breakdowns at T with implant exposure and eight wound dehiscences), six of which required surgical revision. In contrast, only three patients (2.8%) in Group B reported a complication, which was wound dehiscence without implant exposure in all cases. None of the dehiscence required surgical revision. The difference between complication and revision rates was statistically significant.
Conclusions
Separating the implant and the mastopexy dissection planes reduces the implant exposure and the reoperation rate in one-stage augmentation mastopexy.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors https://link.springer.com/journal/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As outlined by Spear [1] and later remarked by Lee [2] and Sanniec [3], the goals of mastopexy differ significantly from those of augmentation mammoplasty: the former is designed to lift and reshape the breast, reducing the surface of the skin envelope, while the latter increases the breast volume, counteracting the surface reduction of the mastopexy. Additionally, the amount of scarring associated with augmentation mammoplasty is typically minimal, whereas mastopexy generally requires larger and less concealable scars. These differences account for a revision rate as high as 23% [4,5,6,7,8,9,10] in one-stage augmentation mastopexy.
Detailed surgical planning, a stepwise approach, and meticulous intraoperative techniques are the key points to minimize the risk of complications and to enhance the results. Nevertheless, implant exposure with subsequent removal represents the most serious local complication, and it is often exacerbated by the wound dehiscence usually presenting at the T-junction in classic inverted-T mastopexy.
This study describes the outcomes of a modified technique for single-stage augmentation mastopexy used for over ten years. We retrospectively compared two single-operator cohorts of patients: one undergoing a classic inverted-T with superomedial pedicle augmentation mastopexy and the other undergoing a modified inverted-T with an extended glandular pedicle augmentation mastopexy in which the glandular flaps are designed to protect the implant from exposure and lower the complication rate. The technique consists in the combination of the subcutaneous glandular dissection performed in vertical [11] and peri-areolar [12, 13] mastopexy, the inferior dermo-glandular flap [14, 15] and the classic approach for augmentation mammoplasty with an incision at the inframammary fold.
Materials and Methods
We retrospectively analyzed consecutive patients undergone augmentation mastopexy between January 2013 and December 2022. All patients were evaluated, planned, and operated on by the same surgeon (M. M.). Every patient signed a detailed and personalized informed consent prior to the operation.
When deciding if mastopexy was needed in addition to implant placement, we typically assessed the position of the nipple-areola complex (NAC) with the arm raised to 90°. Abduction mimics the expected lift from a simple implant placement. If the NAC was less than 1 cm lower than the ideal new position, we planned a circular areolapexy. Therefore, patients undergone circular areolapexy were excluded from the study. If the NAC was more than 1 cm lower than the expected position, we planned an inverted-T mastopexy.
The patients were divided into two groups: Group A, patients receiving augmentation mastopexy following the classic inverted-T technique with a superomedial pedicle; and Group B, patients treated with the personal technique described below. Each implant was placed in a partial, submuscular, dual-plane pocket. The choice between a round or shaped implant was based on the patient’s preferences regarding upper pole fullness and global appearance of the breast. The degree of breast ptosis was evaluated using the Regnault scale [16]. The follow-up protocol was equal for all included patient's drains removal at 2–3 days after surgery and follow-up visits at 7, 15, 30, 180, and 360 days follow-up. The personal technique of the Group B was introduced in patients operated from February 2017, in the attempt of reducing the implant exposure in case of dehiscences at the T-junction.
Demographic and operative data were retrospectively collected from a prospectively maintained database. Patient demographics included age, body mass index, smoking status, history of previous breast surgery. The primary outcomes were overall complications and implant failure rates. The secondary outcomes were infections, wound dehiscences, and capsular contractures rates. The two groups were matched on degree of ptosis, comorbidities, and implant volume. This study was conducted in accordance with the Declaration of Helsinki.
Surgical Technique
With the patient standing, the midline, inframammary fold, and breast meridian are marked. The distance between the sternal notch and nipple is marked. The ideal position of the superior border of the nipple-areola complex (NAC) is then marked on the breast meridian, and the distance is checked for symmetry. A point is marked 5 cm inferior to this point and 2 cm from each side to obtain the three reference points for designing the dome of the keyhole. The markings were then continued at the inferior pole for conservative skin excision. With the arms abducted and skin under tension, the inferior pole height was marked at 7.5 cm and an inferior horizontal excision was planned. The implant was then chosen based on the breast base; projection and volume were adjusted according to the patient′s desire. The NAC position on the most projected point of the implant was considered a fixed point, and the level of the IMF was modified accordingly. (Video 1, Supplementary Digital Content 1).
Intraoperatively, under general anesthesia and with the patient in the supine position, an inframammary incision was placed in the upper half of the planned horizontal lozenge of the inverted-T (Figure 1A). The subpectoral plane was addressed using Ellis′s retractors and blunt elevation with closed Metzenbaum scissors. A dual-plane type II breast pocket was prepared, with soft tissue dissection performed using monopolar electrocautery. A silicon drainage was placed in each pocket, gloves were changed, and the implant was inserted. Every implant received a triple antibiotic immersion prior to its insertion [17]. The lower-pole glandular flap was then sutured to the deep fascia at the level of the new IMF to close the pocket and avoid future dislocation.
Intraoperative details. A The inframammary incision for the implant placement is set at the upper half of the excisional horizontal area in the mastopexy design. B Complete de-epithelization is performed after the implant has been placed and its access closed. C The medial and lateral pillars and elevated in a subcutaneous, supra-glandular plane, similarly to that followed during a mastectomy. D The NAC is lifted, and the pillars are sutured
Full de-epithelization was performed in the area marked preoperatively (Figure 1B). The medial and lateral pillars were then subcutaneously dissected, following the same plane as the nipple-sparing mastectomy (Figure 1C). After full dissection and closure of the upper and lower areolar borders, the dermal strip of the lower pole was incised to consent to the lifting of the NAC (Figure 1D, Video 2 supplementary Digital Content 2). Eventually, the inferior T-point between the inferior pole and IMF was sutured, and all incisions were closed with monofilament absorbable subcutaneous stitches. A mild elastic dressing is applied.
Statistical Analysis
Descriptive statistics were used for demographics, and surgical outcomes data among groups were recorded as frequencies and percentages for categorical variables and as means and standard deviations for numerical variables.
For numerical variables, the T-test was utilized. For categorical variables, differences were measured using the Chi-square and Fisher exact tests. The analyses were performed using SPSS statistics software (IBM, 1 New Orchard Road, Armonk, New York 10504-1722, United States).
Results
A total of 216 female patients were included in this study, 107 in group A and 109 in group B. Median age (28 versus 25 years, respectively) and number of active smokers (9.5 versus 10.5 sig/die, respectively) were not significantly different between the groups (p > .05). No comorbidities were present in the two groups. Most patients in both groups had Grade III breast ptosis (n. 86 versus 77, respectively), without a significant difference. Anatomic implants were used in 86 (80%) patients of the Group A and 87 (79%) patients of the Group B, with a mean volume of 290 cc (± 39) in Group A and 300 cc (± 44) in group B. The median nipple-to-fold length passed from 12 cm preoperatively to 8 cm postoperatively.
The average breast lift was 6 cm for Group A (from a median sternal notch-to-nipple distance of 25 cm to 19 cm) and 5 cm for Group B (median 25–20 cm). The overall average follow-up period was 45 ± 15 months, with no significant difference between the groups (46 months for Group A versus 43 months for Group B, p = .17).
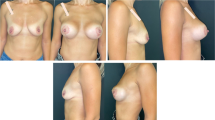
Complications were registered in ten patients (9.3%) in Group A (two wound breakdowns at T with implant exposure and eight wound dehiscences without implant exposure), six of which required surgical revision. In contrast, only three patients (2.8%) in Group B reported a complication, which was wound dehiscence without implant exposure in all cases. None of the dehiscence required surgical revision. One dehiscence in Group A and one in Group B occurred in active smokers. The difference between complication and revision rates in the two groups was statistically significant (p = .045 and .035, respectively). Patient characteristics and outcomes are summarized in Table 1. The results of pre- and postoperative day 30 of the case shown in the surgical technique paragraph are shown in Figure 2. Example cases at 1 year follow-up are shown in Figure 3 for Group A and in Figure 4 for Group B. Example case of a 10 year follow-up for Group B is shown in Figure 5. A complete list of patients and their data are reported in Table 2.
Preoperative (above) and postoperative results at day 30 (below) of the case presented in figure 1
Discussion
Mastopexy and augmentation mammoplasty have opposite surgical goals, with the increase in the breast volume contrasting the skin reduction. Consequently, single-stage procedures reported relatively high frequencies of complications, among which wound dehiscence with implant exposure represents the most serious local complication.
Khanavin et al. [18] performed a systematic review and meta-analysis of the outcomes of single-stage augmentation mastopexy from 23 retrospective cohort studies. They reported a pooled complication rate of 13.1% (6.7–21.3% CI) and a pooled reoperation rate of 10.65% (6.7–15.4% CI). The inverted-T resulted in a lower rate of ptosis recurrence (3.2%). A major limitation of their study was the evident heterogeneity among studies, both in quality and level of evidence; nevertheless, their study is the only meta-analysis available to date on single-stage augmentation mastopexy. With our modifications, combining the approach of three different breast operations, we lowered the complication rate from 9.3 to 3% and the revision rate from 5.6% to none. By integrating techniques from three distinct breast operations, we reduced the complication rate from 9.3 to 3% and eliminated the revision rate, which was 5.6% in Group A. Interestingly, even if three wound dehiscences were registered in the modified technique group, none of them resulted in implant exposure or needed reoperation.
Stevens et al. [9] reported on 615 consecutive patients undergoing single-stage mastopexy. Implant infection with explanation occurred in 0.8%, poor scarring in 5.7%, and wound-healing problems in 2.9% of patients. The global revision rate was 16.9%. The major limitation of their study is the extreme variability of their cohort: they included different procedures (both primary and secondary mastopexy), implant materials (silicone and saline), surgical techniques (circumareolar, vertical, and inverted-T), and pocket location (both sub glandular and submuscular). Messa et al. [10] reported similar outcomes in their 1183 consecutive single-stage augmentation mastopexy cohort. Although, they also included circumareolar, vertical, and inverted-T techniques. In contrast, in our study, we included only a specified type of augmentation mastopexy, reporting a 2.8% of complications with no revisions.
Sanniec et al. [3] reported one of the largest single-operator case series in the literature, with 251 single-stage augmentation mastopexies. In their study, the mastopexy technique was like our inverted-T, including the preservation of the inferior dermal flap at the T; though, the elevation of the two parenchymal pillars was conducted at full thickness, connecting the skin incision directly to the implant pocket. They reported 14% of total complications, 3.6% revisions, and 0.8% implant removal. On the contrary, our modified technique achieved sensibly lower rates and no revisions.
Our modified chimeric technique reunites the advantages of three different approaches in breast surgery. The inframammary incision and submuscular placement of the implant reduce the risk of infection and capsular contracture [19]; the inferior pole de-epithelized flap, beveling the access of the implant pocket from the inframammary part of the inverted-T wound, offers vascularized coverage to the inferior profile of the implant and avoids direct exposure in the case of a wound breakdown. This was partially derived from the Balcony technique described by De Vita et al. [20]. Finally, subcutaneous dissection with elevation of thin and pliable medial and lateral pillar flaps, derived from the peri-areolar and vertical mastopexy techniques, optimizes skin re-draping, and maximizes control over nipple position and lower pole height.
The main limitation of this study stands in the retrospective nature, which does not allow a strict control of confounding biases, even if they had been reduced by the cohort matching, the single operator procedures, the equality among the types of implants, and the degree of breast lift. A prospective study might help confirming that the described technique is able to reduce the rate of complication and revision in augmentation mastopexy.
Conclusions
Our chimeric technique of single-stage augmentation mastopexy improves the safety profile of this intervention, reducing overall complications and minimizing the risk for wound dehiscence and implant exposure.
References
Spear S (2003) Augmentation/mastopexy: surgeon, beware. Plast Reconstr Surg. https://doi.org/10.1097/01.PRS.0000072257.66189.3E
Lee MR, Unger JG, Adams WP (2014) The tissue-based triad: a process approach to augmentation mastopexy. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000000387
Sanniec K, Adams WP (2019) The tissue-based triad in augmentation mastopexy: single-stage technical refinements. Aesthet Surg J. https://doi.org/10.1093/asj/sjz006
Beale EW, Ramanadham S, Harrison B, Rasko Y, Armijo B, Rohrich RJ (2014) Achieving predictability in augmentation mastopexy. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000000079
Stevens WG, Freeman ME, Stoker DA, Quardt SM, Cohen R, Hirsch EM (2015) One-stage mastopexy with breast augmentation: a review of 321 patients. Plastic Surg Complet Clinic Mast PRS Breast Lift. https://doi.org/10.1097/01.prs.0000282726.29350.ba
Kirwan L (1999) Augmentation of the ptotic breast: simultaneous periareolar mastopexy/breast augmentation. Aesthet Surg J. https://doi.org/10.1016/S1090-820X(99)80005-3
Stevens WG, Stoker DA, Freeman ME, Quardt SM, Hirsch EM (2007) Mastopexy revisited: a review of 150 consecutive cases for complication and revision rates. Aesthet Surg J. https://doi.org/10.1016/j.asj.2006.12.014
Rubin JP (2006) Mastopexy after massive weight loss: dermal suspension and total parenchymal reshaping. Aesthet Surg J. https://doi.org/10.1016/j.asj.2006.01.010
Grant Stevens W, MacIas LH, Spring M, Stoker DA, Chacón CO, Eberlin SA (2014) One-stage augmentation mastopexy: a review of 1192 simultaneous breast augmentation and mastopexy procedures in 615 consecutive patients. Aesthet Surg J. https://doi.org/10.1177/1090820X14531434
Messa CA, Messa CA (2019) One-stage augmentation mastopexy: a retrospective ten year review of 2183 consecutive procedures. Aesthet Surg J. https://doi.org/10.1093/asj/sjz143
Ahmad J, Lista F (2008) Vertical scar reduction mammaplasty: the fate of nipple-areola complex position and inferior pole length. Plast Reconstr Surg. https://doi.org/10.1097/01.prs.0000302453.26842.5d
Benelli L (1990) A new periareolar mammaplasty: the “round block” technique. Aesthetic Plast Surg. https://doi.org/10.1007/BF01578332
Austin RE, Saheb-Al-Zamani M, Lista F, Ahmad J (2019) Periareolar augmentation-mastopexy. Aesthet Surg J 39(9):953–965. https://doi.org/10.1093/asj/sjz128
Svedman P (1991) Correction of breast ptosis utilizing a “fold over” de-epithelialized lower thoracic fasciocutaneous flap. Aesthetic Plast Surg. https://doi.org/10.1007/BF02273832
Mansur AEC, Graf RM, Fadul R et al (2020) Simultaneous augmentation mastopexy: an innovative anatomical approach—the fascioglandular flap for improved lower pole support. Aesthetic Plast Surg 44(5):1414–1420. https://doi.org/10.1007/s00266-020-01702-5
Regnault P (1966) The hypoplastic and ptotic breast. Plast Reconstr Surg. https://doi.org/10.1097/00006534-196637010-00004
Awad AN, Heiman AJ, Patel A (2022) Implants and breast pocket irrigation: outcomes of antibiotic, antiseptic, and saline irrigation. Aesthet Surg J. https://doi.org/10.1093/asj/sjab181
Khavanin N, Jordan SW, Rambachan A, Kim JYS (2014) A systematic review of single-stage augmentation-mastopexy. Plast Reconstr Surg 134(5):922–931. https://doi.org/10.1097/PRS.0000000000000582
Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH (2013) Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. https://doi.org/10.1016/j.bjps.2013.04.046
De Vita R, Zoccali G, Buccheri EM (2017) The balcony technique of breast augmentation and inverted-T mastopexy with an inferior dermoglandular flap. Aesthet Surg J. https://doi.org/10.1093/asj/sjx142
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
An information pamphlet was provided to every patient at least one week prior the operation, and written informed consent was obtained on the operation day.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
This video shows the preoperative planning and design for our technique of single-stage augmentation mastopexy. (MP4 61102 KB)
This video shows an intraoperative technical tip. After implant placement and de-epithelization, the medial and lateral pillars are elevated. The central sling of dermis has to be incised, in order to facilitate the lifting of the NAC. (MP4 42476 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marino, M., Alessandri-Bonetti, M., Carbonaro, R. et al. Technical Refinements for Reducing Reoperations in Single-Stage Augmentation Mastopexy: A Retrospective Matched Cohort Study. Aesth Plast Surg (2024). https://doi.org/10.1007/s00266-024-03917-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00266-024-03917-2