Abstract
Background
Current breast implant prevalence within the general population remains elusive. An accurate prevalence is critical to serve as the denominator for any assessment of breast implant-related complication. The purpose of this manuscript is to assess this prevalence in women aged 20–70 years in Italy.
Materials and Methods
Eight reviewers, demonstrating a mean sensitivity of 87.0% and specificity of 97.0%, were recruited for retrospective identification of implants on chest radiographs from a tertiary academic hospital in a major urban setting. Three final reviewers were selected, and they assessed all eligible chest radiographs collected between January and December 2019. The hospital-based population was compared to epidemiological data at a local, regional and national level to demonstrate homogeneity of age structures using the phi correlation coefficient.
Results
We identified 3,448 chest X-rays which yielded 140 implants, with an overall prevalence of 4.1% for women aged 20–70. Implants were bilateral in 76% of cases and unilateral in 24%. They were placed cosmetically in 47.1% cases and used for reconstruction in 52.9% cases. Phi correlation coefficient found no differences across hospital-based, local, regional and national populations.
Conclusion
A validated method was performed to estimate implant prevalence from an academic hospital in a major urban setting at 4.1% and was used to estimate national prevalence in Italy. The implications of this epidemiologic study may reach across national borders for improved understanding of breast implant epidemiology and in predicting the total number of patients within a given population that may be affected by device complications.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The US Food and Drug Administration recently issued several orders to strengthen breast implant (BI) risk communication including restricting implant sales, boxed warnings, mandatory informed consent checklists, and updated surveillance recommendations [1, 2]. In 2021, the scientific committee on health, emerging and environmental risks (SCHEER) of the European Commission released a detailed review of breast implant safety and updated guidance on breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) [3]. An identified gap within the scientific literature was an accurate assessment of national and global breast implant prevalence critical for the determination of complication rates and the extrapolation of clinical outcomes from post-market approval trials. As a result of mass media coverage and public scrutiny, considerable research efforts have been directed toward understanding diseases related to BI, including BIA-ALCL [4, 5]. Despite the numerous efforts, several points remain unanswered, including the BI prevalence in the general population. This represents the denominator necessary to calculate accurate rates of any BI-related sequelae, important to physicians, manufacturers, government authorities, and patients assessing the risks and benefits of these devices.
As a consequence of lack of mandatory National Breast Implant Registries (NBIR) and only few being opt-out, epidemiologic studies on BI prevalence in the scientific literature are few and sparse [6]. Traditional estimation methods include sales reports from implant manufacturers, surgery reports from registered surgeons affiliated with plastic surgery societies, mail-based surveys, and subjective expert opinion [7,8,9,10]. Though straightforward, these methods are flawed, use incomplete datasets and provide inaccurate estimates [11]. Once established, NBIRs will take long before collecting useful data, allowing for unconventional alternatives to emerge. De Boer et al. [12] conducted a study design identifying radiopaque BIs on chest radiographs (Chest X-ray or CXR) to calculate BI prevalence within the Dutch female population. We intended to validate their results by using the same methodology in Italy, a country with a larger female population than The Netherlands (30.8 vs. 8.75 million), and 5th worldwide for cosmetic breast augmentation surgeries [13]. BI prevalence was determined as primary endpoint, while laterality, indication for placement (cosmetic vs reconstructive), and indications for CXR as secondary endpoints.
Materials and Methods
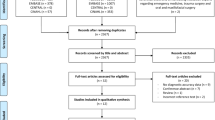
This study classifies as a retrospective observation study, which was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist. It received ethics committee approval (Ref. 7001_2020) and was conducted between April 2020 and June 2021. The study consisted in recognizing the presence of breast implants on CXR to calculate breast implant prevalence. For this purpose, it was conducted in two phases: a preliminary followed by second phase.
Preliminary Study Phase: Identification of Reviewers
This phase consisted in identifying individuals who could consistently and reliably recognize BIs on CXR using predetermined criteria described by de Boer et al. [12], which are given as follows: (1) projection lines that follow the contour of the BI within the breast; (2) calcifications along the periprosthetic capsule; and (3) the typical signs of a metal magnetized valve/port of the tissue expander. This phase was initiated by submitting a 3-hour tutorial, training session and test to eight potential reviewers to assess their sensitivity and specificity in recognizing BIs on CXR. Sensitivity was defined as the ability to accurately assess CXRs as positive for BI, while specificity was defined as the ability to correctly assess CXRs as negative for BI. The training session included the retrospective analysis of 180 CXR of patients who had previously undergone BI surgery (insertion, revision or removal) at our facility in the form of a standardized quiz. Out of 180 radiographs, 60 displayed one or more BIs, while 120 showed none. All CXRs were performed in our center using a remote-controlled direct radiology (DR) system and were collected through our facility’s Centricity Enterprise Web application PACS (Picture Archiving and Communication System) [GE Healthcare, USA]. Radiological images were assessed in dual-headed workstations, with dedicated NEC [Sharp NEC Display Solutions Ltd.] high-luminosity and high-resolution reporting monitors (2,5 K x 2 K), as per usual normal working conditions for radiologists. BI status (i.e., presence or absence) was confirmed by using our prospectively maintained breast reconstruction patients database with a minimum follow-up of 3 years. No medical file was directly accessed, to respect patient confidentiality. The eight potential reviewers all received the same instructions and training, and were later asked to test their skills by responding to a 180-question test using a sample answer sheet. Individual sensitivity and specificity were calculated upon completion, and the three most accurate participants were selected (Table 1).
Secondary Study Phase: Assessment of bi Prevalence
This phase consisted in the large-scale evaluation of BI prevalence in the target population, which was the female population who received CXR at our institution. Inclusion criteria were as follows: women aged between 20 and 70, who received one or more CXRs in our facility, regardless of indication, between 01/01/2019 and 31/12/2019. Only one CXR per patient was included. Exclusion criteria were given as follows: pregnant woman and low-quality CXR. Any disagreement among reviewers was settled during consensus-based meetings, with blinded reevaluation.
An appointed third party was in charge of selecting patients in accordance with inclusion and exclusion criteria. They selected specific identifying codes of all eligible patients from the PACS and stored their radiological images, ensuring the full anonymity of each image before submitting them to the reviewers. All data were manually tabulated in an Excel spreadsheet (Microsoft Office, Albany, USA) later used for calculations. The estimated prevalence of BIs was calculated similarly to what was described in previous studies [12], with the following formula as a function of the presumed true prevalence (p), the specificity (spec) and the sensitivity (sens) of the reviewers:
To determine secondary endpoints, the indication of each CXR was recorded by the third party before the anonymization process. Conversely, laterality and distinction between cosmetic or reconstructive BI indication were made by each reviewer, according to objective signs from the images, such as absence of breast gland and/or nipple, and marked as “unknown” in cases where it could not be determined. Mastectomies were distinguished from aplasia according to age, presence of central lines and eventual hemostatic clips. The homogeneity of age structures of the 4 populations (Sant'Andrea, Rome, Lazio, Italy) was assessed using the phi correlation coefficient, to verify whether data from the large population at a hospital-level could have significant difference with the population at a local, regional and national level.
Results
Preliminary Phase
Reviewers included two fully trained radiologists expert in breast imaging, two plastic surgery consultants, two plastic surgery residents, and two medical students without prior training (Table 1). Median sensitivity of the eight reviewers was 87.0% (range 57.0–96.0%), whereas median specificity was 97.0% (range 90.0–98.3%). The highest scores in terms of sensitivity and specificity were achieved by one radiologist (A), one plastic surgery consultant (B) and one plastic surgery resident (C), who later served for the following phase of the study.
Secondary Phase
After initial assessment, 3537 unique patients aged 20–70 had undergone a CXR at our institution within the selected time frame: 82 (2.3%) were excluded due to artifacts or unreadable diagnostic images. Thus, 3448 CXR were deemed eligible and their images were analyzed. Each patient CXR had a posteroanterior and a latero-lateral view, except for the emergency radiographs, where a single view was taken in most instances. Disagreement was observed in 116 instances (3.4%), and all were solved after consensus-based discussion. Mean age was 52.5 years (ranging from 20 to 70), the population was subdivided into the following groups: 20- to 30-year-olds (246 patients), 31- to 40-year-olds (383 patients), 41- to 50-year-olds (759 patients), 51- to 60-year-olds (940 patients) and 61- to 70-year-olds (1120 patients). Since the sample sizes were very big, the p value is not indicated; therefore, measures of effect size (phi) were calculated. In this study, phi coefficient was next to zero: Sant'Andrea versus Roma: 0.021, Sant'Andrea versus Lazio: 0.018, Sant'Andrea versus Italia: 0.005, which led to the conclusion that the age distribution of the control populations does not significantly differ from the Sant'Andrea age distribution. In fact, with a sufficiently large sample, a statistical test will almost always demonstrate a significant difference, unless there is no effect whatsoever, that is, when the effect size is exactly zero, yet very small differences, even if significant, are often meaningless [14]. Differences in distribution of age populations across populations are detailed in Table 2. Indications for CXR are summarized in Table 3.
A total of 140 women were identified with BI, 106 (76%) bilateral while 34 (24%) unilateral, of which 14 (41%) to the right side and 20 (59%) to the left. Indication for BI placement was “unknown” in 18 (12.9%) women, while in the remaining 122 was cosmetic in 62 (50.8%) and reconstructive in 60 (49.2%) women. Overall mean BI prevalence was 4.1% and varied according to age range groups as 2.1% (20–30 age), 4.4% (31–40 age), 5.2% (41–50 age), 4.9% (51–60 age), and 2.9% (61–70 age) (Fig. 1).
Discussion
Determination of an accurate national BI prevalence is essential for the global health risk assessment. Being silicone radiopaque, the use of CXR on a wide population has emerged as a novel means for estimation of these data, since useful implant registries are missing [15]. Compared to de Boer’s used criteria [12], we added a fourth one which is the presence of radio frequency identification (RFID) transponder (Fig. 2). While implant projection lines were the most common sign to be found, calcifications could only be identified in older patients with a presumably longer time of implant permanence. Magnetized valves/ports were unsurprisingly recognized in only 7 patients (5%) because of the usually transient life of tissue expanders, and the fourth was exclusively associated with specific types of BIs using a novel non-invasive traceability system [16]. Although in breast reconstruction patients who received deep inferior epigastric perforator (DIEP) flap, the latter sign could be confused with a hemostatic metal clip, those are usually multiple, often located in the axilla as well as in linear fashion along the flap pedicle or sparse around the chest area. Conversely, the RFID transponder is single, oval-shaped, radiopaque structures, exclusively found in the region where the implant is located (Fig. 3).
The de Boer et al. criteria for identifying breast implants on chest X-rays. They include projection lines that follow the contour of the implant within the breast, as seen on a posteroanterior (A) and latero-lateral view (B); calcifications along the periprosthetic capsule (C); and the metal magnetized valve/port of a tissue expander (D)
Differences between radio frequency identification (RFID) microchips bilaterally placed in breast implants A in a patient who underwent bilateral implant-based breast reconstruction, and hemostatic metal clips in the axilla and breast B in a patient who underwent left unilateral DIEP flap-based breast reconstruction
Bilateral presence of BI varied across age groups, being higher in the younger population having more frequent cosmetic indication compared to the older having, mostly unilateral implants for reconstructive purposes (Fig. 4). The main limitation from our study is the fact that findings were only collected from a single, albeit large, medical center in Italy, potentially limiting the scalability of prevalence. However, our patient age distribution was compared to large epidemiological data on a regional and national scale, and was deemed statistically representative of Italy. Another limitation is how indication for implant placement could only be identified in 87.1% of patients due to the fact that it had to be determined according to indirect radiological signs.
The difference in BI prevalence compared to de Boer’s findings (respectively, 4.1% vs. 3.0%) may warrant some reflection on the heterogeneity of BI practices from country to country, suggesting a BI use more widespread in Italy than in the Netherlands. This can be confirmed by the International Society of Aesthetic Plastic Surgery (ISAPS) 2019 statistics, ranking Italy 5th worldwide for number of cosmetic breast augmentation surgeries (56,073 procedures) [13], just similar but behind USA, Brazil, Japan, and Mexico. While the Netherlands did not make the top sixteen countries worldwide and is only included in aggregate by ISAPS within Global estimates with 12,419 breast implant procedures, of which 8149 (66%) cosmetic augmentations and 4242 (34%) reconstructive [17]. The Italian female population counts 30.8 million while the Dutch 8.75 million, which means that 0.182% (56,073/30.8 million =) of Italian females vs 0.093% (8149/8.75 million =) of Dutch females received a breast augmentation in 2019 [18, 19]. While these figures only represent rough estimates, they suggest that Italian females underwent nearly twice as many breast augmentations per capita as Dutch females did. Unfortunately, a similar direct comparison between breast reconstruction cannot be made, as data are not readily available in Italy due to the lack of formal Breast Implant Registry, but a comparison can be extrapolated by breast Cancer data. In 2020, the Global Cancer Observatory reports 55,133 new breast cancer in Italy [20], counting 13.3% of all new cancers, while The Netherlands’ 15,725 counting 11.9% of all new cancers. Therefore, with a larger incidence of breast cancers in Italy, it is reasonable to infer that breast reconstruction figures are higher as well. These notions strengthen the importance of an Italian estimate to be adopted in countries with higher BI practice.
Finally, being our 4.1% figure representative of the Italian female population aged 20–70 (20,139,440) [21], we could presume 825,717 women with BI in Italy. As the rest of the European Union of 28 countries (EU-28), counting 170,611,364 women aged from 20 to 70, could not be considered all with high BI practice, neither with low, we could reasonably adopt a mean value of 3.55% prevalence rate, estimating 6.056.703 women with BI compared to 5,118,341 as by de Boer et al.’s calculation.
Their original study presents other differences to be considered compared to ours. In their preliminary phase, they recruited patients who had undergone a CT and/or MRI scan within ± 3 months from the CXR, selecting a brief time frame to minimize the risk that BI status might have changed between the scan and the radiograph. Differently we have selected CXR from patients with a known BI history, hence reducing the bias of uncertainty introduced by scans performed at a different time from the radiograph. Consequently, we found that our reviewers’ sensitivity (87.0%) and specificity (97.0%) exceeded de Boer et al.’s, who reported using a threshold of 70% for sensitivity and 80% for specificity to select theirs. Additionally, our study includes data collected from a large center in Central Italy, with a catch basin representing both rural and urban geographies, whereas de Boer et al. from 2 smaller regional centers in the Netherlands. Conversely, they used epidemiologic data from their Breast Cancer Screening Program to apply their local BI prevalence to the entire country. This was not feasible in Italy due to differences in data collection, and we compared our population with local, regional and national ones and found no significant differences in terms of homogeneity in age distribution.
It was notable that within this study, the mean age was sizably higher than in the Netherlands (52.5 vs. 46.5 years). This is likely not due to differences in life expectancy, which are comparable in both populations: 82.012 years for the Dutch [22], 83.198 for the Italian in 2019 [23], but more likely be due to dissimilar BI indication among both studies. We report that 47.1% of placements were cosmetic and 52.9% were reconstructive, while de Boer et al. found, respectively, 54.3% versus 45.7%. Breast augmentation patients have a lower mean age of 34 years [21], compared to 62 years in breast reconstructive patients [24,25,26]. Scarce presence of young individuals in the population studied is likely due to the fact that they require less frequently CXRs since they are generally fit and healthy [27]. This difference may indicate a possible underestimation of the cosmetic population as large-scale national multicenter data [28] and international findings [29] report that 75% of BI for cosmetic purposes while 25% reconstructive. Had our population included a breast augmentation-to-reconstruction ratio of 3:1, and had this ratio been confirmed for the Italian population, our BI prevalence could have been as high as 6.4%. We are unsure whether our percentage of younger participants was different from de Boer et al.’s because figures regarding age range distributions were not disclosed, thus could not be verified. These elements could explain the reported difference in BI placements, but also highlight the possibility of selection bias, which can only be solved by recruiting more patients from different hospitals in other parts of the country or even beyond national border. This is something we have already begun to work toward in the form of a multicentric study on a European scale.
Despite our best efforts, the use of unconventional methods for calculating BI prevalence will never surpass mandatory reporting in NBIRs [30]. Therefore, we strongly support recommendations of the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER)’s to implement their use [2] for obtaining a better estimate of risks related to BI complications. In Italy, despite the implementation of Law n°86 on the 5th of June 2012 [31] for the creation of regional and national breast implant registries, nearly 10 years since the law’s enactment, the country’s tracking efforts are limited to a pilot study in 2019, with 269 participating surgeons as of September 2021: [32] a number far from the required standards to generate widely applicable data. Therefore, as long as relevant institutions cannot guarantee the establishment of reliable NBIRs, our method appears the most reliable, particularly if validated in countries with different demographic.
Conclusion
Having an accurate breast implant prevalence within the general population informs regulatory efforts, complication risks, and patient informed consent. We applied an already established method to the epidemiologic setting of the Lazio region in Central Italy. The results derived from this study, first of its kind in Italy and largest to date, helped estimating a more accurate BI prevalence in females between 20 and 70 years of age, statistically representative of entire Italy. The utility of this study spreads well beyond its national borders as Italy represents the top 5th breast implant market worldwide. Our figure could serve as a new benchmark denominator for calculating BI-related health hazards and complications for countries with similar breast implant practice to Italy. This methodology will allow greater understanding of global BI epidemiology with nation-specific prevalences through a multicenter project already in its infancy stages.
References
FDA Strengthens Breast Implant Safety Requirements and Updates Study Results. FDA Website. https://www.fda.gov/medical-devices/implants-and-prosthetics/breast-implants. Accesed 27 Oct 2021
Santanelli di Pompeo F, Paolini G, Firmani G, Sorotos M (2022) History of breast implants: back to the future. JPRAS Open 32:166–177. https://doi.org/10.1016/j.jpra.2022.02.004
De Jong WH, Panagiotakos D, Proykova A et al (2021) Final opinion on the safety of breast implants in relation to anaplastic large cell lymphoma: report of the scientific committee on health, emerging and environmental risks (SCHEER). Regul Toxicol Pharmacol 125:104982. https://doi.org/10.1016/j.yrtph.2021.104982
Santanelli di Pompeo F, Sorotos M, Clemens MW, Firmani G (2020) Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): review of epidemiology and prevalence assessment in Europe. Aesthet Surg J. https://doi.org/10.1093/asj/sjaa285
Santanelli di Pompeo F, Clemens MW, Atlan M et al (2022) Practice recommendation updates from the world consensus conference on BIA-ALCL. Aesthet Surg J 42(11):1262–1278. https://doi.org/10.1093/asj/sjac133
Brown SL (2002) Epidemiology of silicone-gel breast implants. Epidemiology 13(Suppl 3):S34–S39. https://doi.org/10.1097/00001648-200205001-00008
Cook RR, Delongchamp RR, Woodbury M, Perkins LL, Harrison MC (1995) The prevalence of women with breast implants in the United States–1989. J Clin Epidemiol 48(4):519–525. https://doi.org/10.1016/0895-4356(94)00208-8
Deapen DM, Pike MC, Casagrande JT, Brody GS (1986) The relationship between breast cancer and augmentation mammaplasty: an epidemiologic study. Plast Reconstr Surg 77(3):361–368. https://doi.org/10.1097/00006534-198603000-00001
Cook RR, Perkins LL (1996) The prevalence of breast implants among women in the United States. Curr Top Microbiol Immunol 210:419–425. https://doi.org/10.1007/978-3-642-85226-8_45
Bright RA, Moore RM Jr (1996) Estimating the prevalence of women with breast implants. Am J Public Health 86(6):891–892. https://doi.org/10.2105/ajph.86.6.891
Santanelli di Pompeo F, Sorotos M, Clemens MW et al (2022) Mortality rate in breast implant surgery: Is an additional procedure worthwhile to mitigate BIA-ALCL risk? Aesthetic Plast Surg. https://doi.org/10.1007/s00266-022-03138-5
de Boer M, van Middelkoop M, Hauptmann M et al (2020) Breast implant prevalence in the dutch female population assessed by chest radiographs. Aesthet Surg J 40(2):156–164. https://doi.org/10.1093/asj/sjz136
Isaps.org. (2020) Countries performing the most popular surgical procedures. [online] https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf Accessed 22 Sept 2021
Sullivan GM, Feinn R (2012) Using effect size-or why the P value is not enough. J Grad Med Educ 4(3):279–282. https://doi.org/10.4300/JGME-D-12-00156.1
Deandrea S, Cavazzana L, Principi N et al (2021) Screening of women with aesthetic prostheses in dedicated sessions of a population-based breast cancer screening programme. Radiol Med 126(7):946–955. https://doi.org/10.1007/s11547-021-01357-5
Munhoz AM, Chala L, Melo G, Azevedo Marques Neto A, Tucunduva T (2021) Clinical and MRI evaluation of silicone gel implants with RFID-M traceability system: a prospective controlled cohort study related to safety and image quality in MRI follow-up. Aesthetic Plast Surg. https://doi.org/10.1007/s00266-021-02355-8
Vrolijk JJ, Becherer BE, Hommes JE, et al. (2020) Dutch breast implant registry (DBIR) Annual Report 2019: Version 2020.01. Leiden (NL): Dutch Institute for Clinical Auditing
Worldometers.info. (2021) Italy Population (2021) - Worldometer. [online] https://www.worldometers.info/world-population/italy-population/. Accessed 21 Sept 2021
Worldometers.info. 2021. Netherlands Population (2021) - Worldometer. [online] https://www.worldometers.info/world-population/netherlands-population/. Accessed 21 Sept 2021
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
European Commission (2020) Eurostat-database on population (Demography And Migration). https://ec.europa.eu/eurostat/web/population-demography-migration-projections/data/database. Accessed 1 August 2020
Data.worldbank.org. (2021) Life expectancy at birth, total (years)-Netherlands | Data. [online] https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=NL. Accessed 22 Sept 2021
Data.worldbank.org. (2021) Life expectancy at birth, total (years)-Italy | Data. [online] https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=IT. Accessed 22 Sept 2021
Santosa KB, Qi J, Kim HM, Hamill JB, Pusic AL, Wilkins EG (2016) Effect of patient age on outcomes in breast reconstruction: results from a multicenter prospective study. J Am Coll Surg 223(6):745–754. https://doi.org/10.1016/j.jamcollsurg.2016.09.003
Noone AM, Cronin KA, Altekruse SF et al (2017) Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomarkers Prev 26(4):632–641. https://doi.org/10.1158/1055-9965.EPI-16-0520
Howlader N, Noone AM, Krapcho M et al (eds) (2021) Cancer statistics review, 1975-2018. Table 1.11: median age of cancer patients at diagnosis, 2014-2018, by primary cancer site, race and sex. National Cancer Institute. Bethesda, MD. Accessed on May 27, 2021. https://seer.cancer.gov/csr/1975_2018/
Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW (2008) The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol 67(2):124–132. https://doi.org/10.1016/j.critrevonc.2008.01.013
Coroneos CJ, Selber JC, Offodile AC 2nd, Butler CE, Clemens MW (2019) US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg 269(1):30–36. https://doi.org/10.1097/SLA.0000000000002990
Heidekrueger PI, Sinno S, Hidalgo DA, Colombo M, Broer PN (2018) Current trends in breast augmentation: an international analysis. Aesthet Surg J 38(2):133–148. https://doi.org/10.1093/asj/sjx104
Becherer BE, de Boer M, Spronk PER et al (2019) The dutch breast implant registry: registration of breast implant-associated anaplastic large cell lymphoma-a proof of concept. Plast Reconstr Surg 143(5):1298–1306. https://doi.org/10.1097/PRS.0000000000005501
Italian Ministry of Health (2012) Law n°86, June 5 2012 [in Italian]. [online] Trovanorme.salute.gov.it. https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=42979. Accessed 24 Sept 2021
Campanale A (2021) National breast implant registry-Impact on the plastic surgeon's activity. In: 69th National Conference of the Italian society of plastic reconstructive and aesthetic surgery (SICPRE), Web Edition
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Corresponding author would like to disclose that they received reimbursements for travel/lodgment expenses from the SCHEER-WG in 2019, 2020 and 2021. They have no ownerships or investments to disclose. All other authors hereby certify that to the best of their knowledge, no financial support or benefits has been received, neither by themselves directly, nor by any member of their immediate family or any individual or entity with whom or with which they may have a significant relationship from any commercial source which is related directly or indirectly to the scientific work which is reported on in the article. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Human or Animal Rights
This study received ethics committee approval from Sapienza University (Ref. 7001_2020). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, informed consent was prepared and obtained in accordance with ethics committee standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santanelli di Pompeo, F., Firmani, G., Paolini, G. et al. Determining Breast Implant Prevalence: A Population Study of Italian Chest Radiographs. Aesth Plast Surg 47, 957–965 (2023). https://doi.org/10.1007/s00266-023-03290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03290-6