Abstract
Background
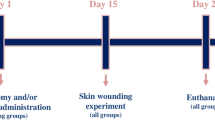
It has been shown that estrogens have a protective effect with regard to tissue ischemia. Therefore, in this macroscopic and histological investigation, the effect of estradiol benzoate on skin flap viability was studied in sham-operated and ovariectomized Sprague-Dawley rats.
Methods
Three months prior to flap surgery a group of rats underwent ovariectomy, while the remaining animals underwent a sham operation. Subsequently, all rats had a 2 × 8-cm skin flap created on the dorsum. Rats were randomly divided into estradiol- or saline-treated groups. Treatment started either on the day of flap excision or 3 days prior to the surgery.
Results
Our results showed that administration of estradiol benzoate prior to and after flap surgery significantly decreases skin flap necrosis in both sham-operated and ovariectomized rats, with the highest survival rate in animals where treatment started 3 days prior to flap surgery.
Conclusion
In conclusion, the observed protective effect of estradiol on skin flap viability could potentially be applied to plastic and reconstructive surgery in postmenopausal women. Nevertheless, further research is needed to explain the exact underlying mechanism and to find the optimal treatment protocol for human clinical practice.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.




Similar content being viewed by others
References
Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM (2005) Helium-neon laser in viability of random skin flap in rats. Lasers Surg Med 37:74–77
Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ (2000) The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg 53:234–239
Holzbach T, Taskov C, Henke J, Busch R, Gänsbacher B, Biemer E, Giunta RE (2005) Evaluation of perfusion in skin flaps by laser-induced indocyanine green fluorescence. Handchir Mikrochir Plast Chir 37:396–402
Mokrý M, Gál P, Harakalová M, Hutnanová Z, Kusnír J, Mozes S, Sabo J (2007) Experimental study on predicting skin flap necrosis by fluorescence in the FAD and NADH bands during surgery. Photochem Photobiol 83:1193–1196
Gümüş N, Odemiş Y, Yılmaz S, Tuncer E (2012) Effect of topically applied minoxidil on the survival of rat dorsal skin flap. Aesthetic Plast Surg 36:1382–1386
Bailet JW, Hoffman LF, Trachy RE, Weymuller EA Jr (1994) The effect of nifedipine on skin flap survival in rats. Laryngoscope 104:253–258
Armstrong M Jr, Kunar DR, Cummings CW (1993) Effect of pentoxifylline on myocutaneous flap viability in pigs. Otolaryngol Head Neck Surg 109:668–675
Freitas FA, Piccinato CE, Cherri J, Marchesan WG (2010) Effects of pentoxyfilline and heparin on reperfusion injury island skin flaps in rats exposed to tobacco. J Surg Res 164:139–145
Ulusoy MG, Uysal A, Kocer U, Karaaslan O, Cuzdan SS, Ayyildiz A, Ustun H (2005) Improved flap viability with sitespecific delivery of sildenafil citrate using fibrin glue. Ann Plast Surg 55:292–296
Richards L, Lineaweaver WC, Stile F, Zhang J, Zhang F (2003) Effect of hyperbaric oxygen therapy on the tubed pedicle flap survival in a rat model. Ann Plast Surg 50:51–56
Findikcioglu F, Findikcioglu K, Yavuzer R, Lortlar N, Atabay K (2012) Effect of preoperative subcutaneous platelet-rich plasma and fibrin glue application on skin flap survival. Aesthetic Plast Surg 36:1246–1253
Takikawa M, Sumi Y, Ishihara M, Kishimoto S, Nakamura S, Yanagibayashi S, Hattori H, Azuma R, Yamamoto N, Kiyosawa T (2011) PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J Surg Res 170:e189–e196
Costa MS, Pinfildi CE, Gomes HC, Liebano RE, Arias VE, Silveira TS, Ferreira LM (2010) Effect of low-level laser therapy with output power of 30 mW and 60 mW in the viability of a random skin flap. Photomed Laser Surg 28:57–61
Emmerson E, Hardman MJ (2012) The role of estrogen deficiency in skin ageing and wound healing. Biogerontology 13:3–20
Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (2010) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870
Mizukami Y (2010) In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J 57:101–107
Toutain CE, Brouchet L, Raymond-Letron I, Vicendo P, Bergès H, Favre J, Fouque MJ, Krust A, Schmitt AM, Chambon P, Gourdy P, Arnal JF, Lenfant F (2009) Prevention of skin flap necrosis by estradiol involves reperfusion of a protected vascular network. Circ Res 104:245–254
van Boxtel R, Cuppen E (2010) Rat traps: filling the toolbox for manipulating the rat genome. Genome Biol 11:217
Xu R, Ge J, Lei Y, Lu X (2009) Improvement effect of estrogen on flap reperfusion injury and blood supply. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23:964–968
Spanholtz TA, Theodorou P, Holzbach T, Wutzler S, Giunta RE, Machens HG (2011) Vascular endothelial growth factor (VEGF165) plus basic fibroblast growth factor (bFGF) producing cells induce a mature and stable vascular network–a future therapy for ischemically challenged tissue. J Surg Res 171:329–338
Huang N, Ashrafpour H, Levine RH, Forrest CR, Neligan PC, Lipa JE, Pang CY (2012) Vasorelaxation effect and mechanism of action of vascular endothelial growth factor-165 in isolated perfused human skin flaps. J Surg Res 172:177–186
Lin J, Steenbergen C, Murphy E, Sun J (2009) Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120:245–254
Etgen AM, Jover-Mengual T, Zukin RS (2011) Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications. Front Neuroendocrinol. 32:336–352
Suzuki S, Brown CM, Wise PM (2009) Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol 30:201–211
Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM (2009) Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids 74:555–561
Gál P, Toporcer T, Vidinský B, Mokrý M, Grendel T, Novotný M, Sokolský J, Bobrov N, Toporcerová S, Sabo J, Mozes S (2008) Postsurgical administration of estradiol benzoate decreases tensile strength of healing skin wounds in ovariectomized rats. J Surg Res 147:117–222
Novotný M, Vasilenko T, Varinská L, Smetana K Jr, Szabo P, Sarišský M, Dvořánková B, Mojžiš J, Bobrov N, Toporcerová S, Sabol F, Matthews BJ, Gál P (2011) ER-α agonist induces conversion of fibroblasts into myofibroblasts, while ER-β agonist increases ECM production and wound tensile strength of healing skin wounds in ovariectomised rats. Exp Dermatol 20:703–708
Acknowledgment
The authors thank B. J. Matthews, MD (Royal Hallamshire Hospital, Sheffield, UK), for his editorial help in preparing the manuscript. This study was supported in part by the Grant Agency of Ministry of Education, Science, Research, and Sport of the Slovak Republic (VEGA No. 1/1095/11), by the Charles University in Prague (project for support of specific university student research No. 266 513) by institutional student grants of the P. J. Šafárik University in Košice (VVGS-39/12-13).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasilenko, T., Slezák, M., Novotný, M. et al. Pre- and/or Postsurgical Administration of Estradiol Benzoate Increases Skin Flap Viability in Female Rats. Aesth Plast Surg 37, 1003–1009 (2013). https://doi.org/10.1007/s00266-013-0151-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0151-z