Abstract
In mammals, birth mass is an important predictor of early growth and survival. Within litters, heavier siblings are usually able to outcompete smaller siblings and gain more resources, thereby often permanently shaping phenotypic development. Early body size and growth are particularly important for later fitness. Only few studies investigated if and how differences within the early family environment contribute to long-term variation in fitness among individuals. We quantified if initial differences in size translate to size differences in adulthood and whether birth mass, relative size within the litter, litter size or the litter sex-ratio affect maturation and reproductive output of female wild cavies (Cavia aperea). Initial differences in mass were maintained until animals reached maximum adult mass at two years of age. Heavier sisters matured earlier and invested more into their first litter than smaller sisters, presumably because smaller sisters invested more into their own growth during the first pregnancy. Growing up in mixed-sexed litters in comparison to female-only litters slowed down maturation in smaller but not the heaviest female within a litter and had no effect on female reproductive effort. Variation in reproduction of multiparous females was to a lesser extent explained by the initial relative size of siblings. Offspring survival to independence was high but slightly lower when mothers had been born as smaller sisters. Our results demonstrate that factors of the early family environment not only affect immediate offspring development but lead to long-term fitness consequences.
Significance
Environmental conditions during an individuals’ early life can have profound long-term consequences. For many animals, siblings represent one of the most important components of the early social environment during development, with larger siblings typically being competitively superior. We here show for the cavy (Cavia aperea) that such early sibling differences affect the timing and investment into first reproduction and potential life-long fitness in a precocial mammal. Initial size differences between siblings persist long into adulthood. Heavier sisters matured earlier than their smaller sisters and invested more into the first litter. Initial size differences among siblings even explained variation in later reproduction. In conclusion, the slight advantage gained by larger relative size within a litter explains a substantial part of individual differences in lifetime fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In polytocous mammal species, birth mass is a good predictor of early growth and survival, with heavier young relative to their littermates often able to obtain a greater share of important resources such as mother´s milk (Fey and Trillmich 2008) or a better position in the litter huddle (Bautista et al. 2008). Such silver-spoon effects are sometimes assumed to translate into individual advantages later in life. Studies investigating such potentially fitness-relevant characteristics on the individual level may ultimately help to understand between-individual variation in fitness, which is present in virtually all vertebrate species (Darwin 1859; Endler 1986; for birds see: Mock and Parker 1997; Drummond 2006).
Litter-specific characteristics such as litter size or litter sex ratio have been observed to shape later phenotype and reproductive fitness. In European rabbits, males from smaller litters, which consequently had higher nestling body masses, were more successful in intra-specific conflicts during their first breeding season (Rödel and von Holst 2009). Male bias in litters is well-known to masculinise females in utero due to high levels of androgens (Saal 1989; Ryan and Vandenbergh 2002). Such masculinised females often delay maturation, e.g., in yellow-bellied marmots (Marmota flaviventris) or European rabbits (Monclús et al. 2014).
The presence of littermates is an influential feature of the early environment in mammals. Littermates can have immediate advantages, for example for thermoregulation (Bautista et al. 2008; Rödel et al. 2008a), or by increasing parental investment in a litter (Hudson and Trillmich 2008). The presence of siblings can have long-lasting effects on individual phenotypic characteristics, including morphological, physiological and behavioural aspects (Hudson and Trillmich 2008). However, litter-mates also compete for limited resources such as mother´s milk or warmth (Bautista et al. 2005; Fey and Trillmich 2008) and sometimes this can result in fights that can end in the death of one or more weaker litter-mates (White 2002). In various callitrichine primates, litter size showed strong effects on survival and reproduction, with increasing survival probability as litter size decreased (McCoy et al. 2019).
Less obvious effects of within-litter competition may result in lower growth rates of smaller offspring (Zepeda et al. 2019) and may even affect later reproduction. Studies in humans, for example, have shown that the presence of elder siblings increased survival chances until maturation but reduced reproductive success thereafter (Nitsch et al. 2013). In non-human mammals, such effects are studied less often, probably due to the inherent difficulties of observing parent-offspring or sibling interactions of mammalian offspring shortly after birth. One study investigating the effects of litter sisters on reproduction, found that female European rabbits started to breed earlier in their first breeding season when a sister was present during early life development (Rödel et al. 2008b).
Here, we investigate the fitness consequences of several litter characteristics on short- and long-term life history traits of individuals. We use data from a captive population of the medium-sized, precocial wild cavy (Cavia aperea). Cavies have a comparatively long pregnancy (60–62 days) and pups are born highly mobile, able to follow the mother within minutes after birth (Rood 1972). In contrast to many other mammals, pups are able to thermoregulate shortly after birth and to supplement maternal milk with solid food within the first two days of life (Rood and Weir 1970; Künkele and Trillmich 1997). Nevertheless, maternal influences such as offspring-directed behaviour (Guenther and Trillmich 2023), maternal age (Trillmich et al. 2019), or litter size (Rehling and Trillmich 2007), are known to influence pup phenotypic development, sometimes long-lasting.
In previous studies, we have shown that siblings of the same litter differ consistently in their immediate phenotypic development until weaning, with heavier pups within a litter expressing a more explorative and active behaviour three days after birth as well as at weaning (Guenther and Trillmich 2015). These behavioural differences were tightly linked to several physiological differences among litter siblings (Guenther and Trillmich 2015). While growth-rates to weaning did not differ among siblings of different size-ranks (i.e., size rank 1 = being born as heaviest, size rank 2 = intermediate or size rank 3 = smallest sibling), smaller siblings had higher blood cortisol concentrations and elevated resting metabolic rates (Guenther and Trillmich 2015). After weaning and separation from their mother and siblings, these phenotypic differences did not persist after maturation (Kraus et al. 2020). Cross-fostering of pups to a smaller or larger size rank directly after birth did, however, not result in an immediate adjustment of the phenotype, potentially indicating some constraints that may persist until later in life (Kraus et al. 2020).
The objective of this study was to assess potential short-and long-term fitness benefits and costs of such constraints caused by early individual development in female wild cavies. Therefore, we first confirmed that the size rank at birth predicts the adult size rank of two-year old, fully grown, non-reproductive animals. Next, we considered potential effects of litter size, litter sex ratio, birth mass and size rank within the litter on the timing of maturation, reproductive effort and success in the initial litter as well as long-term across a six-months breeding period, potentially allowing for three litters. To achieve similar growth rates to weaning, smaller pups of a litter have elevated resting metabolic rates and show higher concentration of metabolism-related hormones such as cortisol (Guenther and Trillmich 2015), potentially indicating a higher investment into growth and self-maintenance compared with heavier litter mates. From this observation, we expect smaller females (i.e., females with a higher size rank), to mature later than their heavier sisters. Similarly, we might expect smaller sisters to invest less effort into their first litter to maintain their own individual growth while bearing their first litter (Raffel et al. 1996). We here test whether such effects translate to later reproductive events, thus potentially affecting life time reproductive success. To achieve similar lifetime fitness, smaller sisters should be able to catch up in reproductive output or live and reproduce longer than their heavier sisters.
Methods
Animals and housing conditions
The animals used for this study derived from the breeding stock of the University of Bielefeld. Cavies were kept and bred in Bielefeld between 1981 and 2018. Originally, animals were caught in Uruguay and Argentina and fresh animals were imported every few years to avoid potential effects of inbreeding. The data presented here were collected across multiple years (2010–2016). As the data reported here were originally collected in the context of studies with other aims, data collection was totally blind with respect to the hypotheses tested in this paper. All focal animals were originally born and grew up until weaning under indoor housing but were later kept and bred in outside enclosures. Hay, pellet food (Firma Höveler, Germany) and water were available ad libitum in both housing conditions. Drinking water was supplemented with vitamin C (1 g/l) once a week and animals received fresh greens (cucumber, carrots, apples etc.) every other day.
Indoor housing conditions
Standard indoor enclosures were of 0.8 m2, equipped with bedding, a shelter for hiding, a rough stone, food and water. Enclosures were located in temperature and light controlled climate chambers at 20 °C ± 2°C and 12:12 L: D. To breed experimental animals, we put two unrelated females together in one enclosure three to four weeks before introducing males for breeding. If the females showed signs of aggressive behaviours, they were separated but kept with auditory and olfactory contact to other females. During breeding (pregnancy and lactation), we exposed them to an increasing photoperiodic regime indicative of spring conditions for the following reasons: In previous studies we showed that cavies react to seasons in various traits such as timing of maturation (Trillmich et al. 2009; Guenther et al. 2014a), reproductive traits (Rübensam et al. 2015), and physiological and behavioural traits (Guenther et al. 2014b). One week before males were introduced to the female enclosures, the light: dark regime was set to 9.5:14.5 L: D followed by an increase of light every ninths day by 15 min. Pregnancy in cavies lasts 60–62 days, thus, starting 58 days after introducing the males, we checked for new-born offspring on a daily basis. All offspring were weighed, sexed and individually marked by haircut at birth and assigned a size rank. The size rank is based on the weight at birth (relative size rank within litter). The pup born as heaviest was assigned to size rank 1, intermediate pups were assigned size rank 2 and the smallest pup was assigned size rank 3. They were left with their mother and siblings until they reached an age of 21 days. Then, they were marked with pit tags (TROVAN, passive transponder system) to ensure permanent individual recognition.
Outside housing conditions
For several experiments, animals were moved to outside enclosures after weaning or later in life (see details below). Outdoor enclosures were approximately 15 m2 in size and consisted of two main parts. A hut of approximately 2 m2 offered shelter from wind and rain. This hut was equipped with bedding, 2–5 smaller shelters, depending on the number of animals living in the enclosure and food and water was located in the hut. During cold months, a heat lamp prevented freezing. The outside part of the enclosure was covered with sand and was exposed to natural light and weather conditions. Two groups of six enclosures were located next to each other, thus animals in adjacent enclosures had visual, olfactory and acoustical contact with each other.
Experimental procedures
Consistency of size rank in litter
To test if birth mass and/ or the size rank assigned at birth translates into later adult body mass (i.e., if the largest sibling in a litter will also be the largest in adulthood), we investigated the consistency across two years. In total, 77 (32 females, 45 males) pups were born in 35 litters (average litter size 2.8 pups). All animals were sexed and individually marked by haircut at birth, weighed and assigned a size rank as described before. After weaning at 21 days of age, animals were kept in unrelated same sex pairs until they were six months old. During this time, they were kept indoors in standard enclosures and participated in various behavioural experiments. At the age of six months, the animals were transferred to outside enclosures. Males were kept singly to prevent aggression but with visual and olfactory contact to other animals. Females were kept in groups of two to five individuals. All animals were taken indoors for breeding (in total ∼ four months for females until after they had weaned their litter but only one month for males to prevent post-partum mating) when they were around a year old and afterwards were again kept in outside enclosures. The animals that were still alive (70 in total) were weighed again when they reached an age of two years, which is the time when they have reached maximum body mass and size (Trillmich et al. 2019). Based on these two-year mass measurements, individuals were again assigned a size rank compared to their siblings. Litters where only one of the animals had survived to this time were excluded (N = 1). We also excluded litters in which more than two animals had died. This left 66 animals from 30 litters for further analysis.
Size rank effects on reproductive traits
Female onset of sexual maturation
To assess the effects of the early family environment on the onset of sexual maturation, data from 73 females born to 37 mothers were used. These data were derived from datasets of earlier studies investigating seasonal and/or social effects on sexual maturation (Trillmich et al. 2009; Guenther and Trillmich 2013; Finkemeier et al. 2016). Earliest reported maturation is with 19 days of age but maturation, especially of females born into autumn or autumn-like photoperiods can frequently be delayed for up to 120 days (Trillmich et al. 2009; Guenther et al. 2021). For females born into spring or spring-like photoperiods such as in this study, the average timing of maturation is 50.4 ± 14.3 days of age (Guenther and Trillmich 2013). To determine the day of maturation, young females were checked daily for opening of the vaginal membrane from postnatal day 10 on. Opening of the vaginal membrane indicates the onset of maturity (Touma et al. 2001). Females were kept in unrelated same-sex pairs from weaning (day 21) onwards. They were located in standard enclosures indoors and experienced a photoperiod of 12:12 L: D until all females had reached sexual maturity.
Reproduction in primiparous females
To determine whether the early family environment affects reproduction of young, not mature, inexperienced females, 22 females born in 14 litters were allowed to breed directly after weaning. These females were kept together with a mature male from day 21 until they successfully produced a first litter. Two females and one male, each, were kept in outdoor enclosures. The breeding started at the end of March (corresponding to spring in Germany) and lasted in total six months. We measured the number of pups born, the number of pups surviving until weaning, the pup birth mass and subsequent growth of pups as well as the maternal effort (litter mass/ mass of the mother at parturition) of the first litter. Females of this species mature early in the growth phase and still grow rapidly at comparable rates than non-pregnant females without reported negative side-effects (Raffel et al. 1996). In total, we had 19 litters (i.e., 19 first litters from different mothers) with surviving offspring that contributed to the statistical analyses. Two of the remaining females did not produce a litter within the six-month observation period and one female had only still-born offspring.
Reproduction in multiparous females
In addition, we monitored the reproductive success measured as the maternal effort per litter, offspring survival and growth to weaning of 33 adult females (age 8–9 months at the start of breeding) which already had bred once before (i.e., they were experienced breeders). All females were kept in outdoor enclosures either alone or in pairs of unrelated females together with an adult male. The breeding started at the end of March and continued for six months throughout the spring/summer period. Fourteen months equals average survival times under natural conditions of the closely related Cavia magna (Kraus et al. 2005), thus our observation period reflects expected life-time reproductive success. All females were kept in unrelated same-sex pairs before the start of the experiment to ensure that none of the females were pregnant or lactating. We determined the number of offspring born, the number of offspring that survived until weaning, their birth mass, growth rate to weaning as well as the number of litters produced during this time and the maternal effort per litter. All females produced between one and three litters (Npups = 275) during the experiment.
Statistical analyses
All data analyses were done using the free software R (version 4.3.0, R Core Team 2023). To analyse the consistency of the birth mass or the size rank at birth and adult (two years) mass/ size rank, we ran linear models with birth mass and the size rank at birth, respectively, as predictor variables.
To test if birth mass, size rank, litter size or litter sex composition (brothers in the litter yes/no) affected the timing of maturation, we ran six linear mixed effects models (packages lme4 (Bates et al. 2014) and lmerTest (Kuznetsova et al. 2017)) each including as fixed effects one of those variables or, at maximum, two variables which were not correlated with each other. Maternal identity (= litter identity) was included as a random effect. Most predictor variables such as birth mass and size rank showed at least a medium correlation, thus, we decided to use AIC comparison among candidate models to find the best predictors. We included a null-model into the comparison leading to a total of seven models for the AIC comparison. The best model or models were the ones with lowest AIC values and with a ΔAIC ≥ 4 to next best model (package AICcmodvg, Mazerolle 2023).
To model maternal effort, pup growth to weaning, litter size, sex ratio of the litter and mortality of pups, we used a similar approach. We constructed five candidate models (including the null-model) using maternal litter size, sex composition of the maternal litter, birth and wean mass as well as size rank of the mother as predictor variables. For reproduction of young females in which only one litter per female was present, we used linear models (lm). In the dataset for adult females, in which females had between one and three litters, we ran linear mixed effects models (lmer) and additionally included litter number (parity) as fixed and maternal identity as random effect. We compared the explanatory power of our set of candidate models by using AIC comparison.
For all models that the AIC comparison identified as the best model or models with similar explanatory power, we next checked the percentage of variance explained (R2 for lm and conditional R2 for lmer, package MuMIn, Bartoń 2023). We considered a model as meaningful only if it differed from the null-model.
Model assumptions of normality were checked visually using qq-plots and heteroscedacity was checked by plotting residuals against fitted values. The plots were created with the package ggplot2 (Wickham 2016) and gridExtra (Auguie et al. 2017).
If not otherwise stated, the values given in the text are mean values with their corresponding standard deviations.
Results
Does the size rank or weight at birth predict the size rank or weight at adulthood?
The weight at birth predicted the weight at two years of age for males and females (N = 66; R2 = 0.13, p < 0.01). When taking sex into account, the explained variance increased to 43% (R2 = 0.43, p < 0.01, Fig. 1a). The size rank at birth, however, predicted the size rank at two years of age even more strongly (R2 = 0.46, p < 0.01). Likewise, when taking sex into account, the explained variance increased to 49% (R2 = 0.49, p < 0.01; Fig. 1b).
Weight at birth (a) and size rank at birth (b) as predictors for weight and size rank, respectively, at two years of age. Shown are the raw data with 95% confidence interval. Black squares and dark grey confidence interval represent males, lighter grey dots and light grey confidence interval represent females
Which early influences predict the onset of maturation?
The timing of maturation for the female cavies in our dataset ranged from 21 to 90 days (38.5 ± 16.4 days). In the best model (Table SI1), the size rank at birth, the sex composition of the litter and the interaction between them predicted 16% of the variation, i.e. 9.66 days, in the onset of maturation.
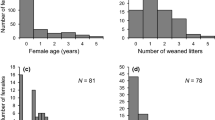
Differences between size ranks in age at maturation depended on the sex-ratio of the litter, and here mainly whether brothers were in the litter or not. In female only litters, females with size rank 1 and 2 matured at around the same time (average maturation: size rank 1 = 35.8 ± 15.6 days, size rank 2 = 39 ± 14 days; Fig. 2a). However, females of size rank 3 tended to show a later onset of maturation (size rank 3 = 42.6 ± 10.2 days; Fig. 2a).
(a) Onset of maturation for females of different size ranks (size rank 1 - dark grey; size rank 2 - mid grey; size rank 3 - light grey) that were born in female only litters or mixed sex litters. (b) Maternal effort for young females calculated as (litter mass/maternal mass)*100 for three different size ranks. Shown are the raw data. Boxes indicate the inter quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5*IQR. Numbers above the box plots indicate the sample sizes
In mixed-sex litters, females with size rank 2 showed a delayed onset of maturation compared to females of size rank 1 (size rank 1 = 32.4 ± 9.2 days, size rank 2 = 45.2 ± 12.5 days; Fig. 2a).
Do early influences affect reproductive traits in primiparous females
Although the null-model was amongst the best models explaining how much a primiparous young female invested into offspring (maternal effort), her birth mass explained 19% (R2m = 0.19) and her size rank in that litter 32% of the variance (R2m = 0.32, Fig. 2b; Table 1). Litter size was best predicted by maternal birth mass (R2m = 0.18) and the maternal size rank in litter (R2m = 0.33, Table 1). Larger females (generally and in relation to litter siblings) had higher maternal effort and larger litters than smaller sister.
Our predictors (litter size, size rank in litter, sex composition of the litter) explained less than 9% of the variance in litter sex ratio, offspring survival and offspring growth to weaning, all of which was not significantly different from a null-model (Table 1). Pup survival was generally very high. In total only 2 out of 31 pups died.
Do early influences affect reproductive traits in multiparous females?
In order to determine the influence of the early family environment on later reproduction, we looked at the reproductive output of multiparous females across six months.
Of the 33 females, 12 were of size rank 1, 12 of size rank 2 and 9 of size rank 3. In total, 20 females gave birth to 3 litters, 10 females had 2 litters and 3 females had only 1 litter during the six months. While all mothers survived, offspring mortality was at 12.7%, i.e., 35 pups out of 275 died.
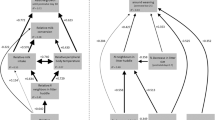
For maternal effort and offspring growth, the best models (Table 2), explained 30% (maternal effort), 36% (offspring growth) of variance and included the predictors maternal size rank at birth, sex composition of the maternal litter, their interaction (Fig. 3) and litter number, i.e., parity (Fig. 4). While maternal effort was similar for females of different size ranks in all female litters, intermediate sisters had lower maternal effort if they had brothers in the litter (mean maternal effort in all female litters = 37.5 ± 1.2, mean maternal effort for intermediate sisters that had brothers = 31.5 ± 2.8, Fig. 3a). Offspring growth on the other hand was similar across females of different size ranks when they had brothers while, in all-female litters, offspring of smaller sisters grew less (Fig. 3b).
Effects of the sex composition of the maternal litter on (a) maternal effort across consecutive litters and (b) offspring growth rates of females from different size ranks. The maternal effort (a) is calculated as (litter mass/maternal mass)*100. Number above the box plots indicate the number of litters. Offspring growth rate (b) represents the daily weight gain between birth and weaning (post-natal day 21). Number above the box plots indicate the number of pups. Boxes indicate the inter quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5*IQR. Plots show the raw data
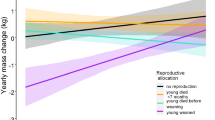
Patterns of consecutive litters (called A, B, C) concerning (a) maternal effort (calculated as (litter mass/maternal mass)*100); (b) the litter sex-ratio (0 - only females in the litter and 1 - only males in the litter) and (c) offspring mortality until weaning with zero meaning that no mortality occurred. Boxes in figure (a) indicate the inter quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5*IQR. Numbers above the box plots indicate the sample sizes
7% of variance in maternal effort was explained by an increase across consecutive litters (Table 2; Fig. 4a). With increasing parity, the sex-ratio of litters became more male-biased (Fig. 4b), explaining a small but significant proportion (4%) of variance in sex-ratios. Parity number explained 14% of variation in offspring mortality to weaning (Fig. 4c).
Discussion
We show evidence that the size rank within a litter and, when investigated separately for the sexes, the birth mass, influence adult mass at two years of age in a precocial mammal that can supplement early energy intake from milk by feeding on solid food within the first days of life. Furthermore, we provide evidence for long-term effects of the early competitive environment within the litter on subsequent reproduction.
Earlier studies investigating the effects of birth mass in general or in relation to litter siblings on subsequent growth and reproduction reported mixed results and focused mostly on altricial species. While birth mass of male bank voles (Myodes glareolus) predicted adult mass well for example, females showed no such relationship under semi-natural conditions (Ylönen et al. 2004). Juvenile body mass of male European rabbits (Oryctolagus cuniculus), also correlated with male body mass at the end of the first vegetation period (Rödel and von Holst 2009). Similar to those studies on altricial species, in cavies birth mass predicted adult body mass when analysed separately for males and females although these animals rely much less on maternal milk supply than the aforementioned altricial species. Relative differences in size within the litter were likewise maintained across two years and explained a similar proportion of variance as sex-specific birth mass. Since body mass is closely related to fecundity, especially in females, this finding is likely important for the animals’ further life history decisions.
For small, short-lived rodents, early maturation is of prime importance for high lifetime reproductive success (Caswell and Hastings 1980; Dobson and Oli 2001; Oli and Dobson 2003). High birth mass has been considered an advantage to mature early in altricial mammals because less resources need to be invested to reach reproductive body mass (Ylönen et al. 2004). Here, we find a similar effect in a precocial species: females born as largest sibling in their litter, mature earlier. Under natural conditions, age of first conception in the closely related dark-backed cavy (Cavia magna) varied between 30 and 321 days and only ∼ 19% of the heaviest females matured and conceived successfully within the reproductive season of their birth (Kraus et al. 2005). No data on maturation age exist for C. aperea, however, maturation ages ranging between 19 and 120 days of age (with 120 days being an artificial observation cut-off) under captive maintenance with ad libitum food and protection from frost, suggest a similar range for the species being studied here.
Also, when it comes to investment into the first reproductive output, being large at birth overall but also in relation to littermates, confers fitness advantages. Larger females were able to put more effort into the first litter. Bank vole females born heavy, likewise produced larger litters in their first reproduction (Koskela 1998). In addition, they had higher reproductive success in favourable environments across several litters when densities were low and food availability high but not during less favourable conditions with food scarcity later during the season (Ylönen et al. 2004). We here saw that female cavies which were born as largest in their litter had higher maternal effort during their first reproductive event but also as experienced breeders and across several litters (mostly two to three litters). Under natural conditions, female Cavia magna produced on average three litters per year which, given their survival rates, constitutes lifetime reproductive success (Kraus et al. 2005). For C. aperea, reproduction data are available from a semi-natural enclosure experiment in Argentina at 29°S. Of 33 young adult females that were released in spring/ early summer into enclosures, 30% survived the winter until the end of the experiment 213 days later. Average survival time was 118 days (< 21–213 days). The average number of litters produced per surviving female was 2 (1–3 litters) (Sobrero and Eberhardt, unpubl. data).
In primiparous females, neither the size rank of mothers nor their birth mass affected offspring survival to weaning or offspring growth rate until weaning. However, in multiparous females, offspring of mothers that themselves had been born large compared to their littermates, had slightly improved survival. Offspring growth to weaning was also affected by the maternal rank within the litter but not by birth mass in multiparous females. Offspring of mothers that had been born as the largest pup in their litters had higher daily weight gain than offspring of smaller mothers. The difference in reported effects between primiparous and multiparous females was likely mediated by differences in the size of their respective litters. Litters of primiparous females were small (LS: 1.9 ± 0.5) while multiparous females had larger litters on average (LS: 3.3 ± 1.5). While offspring mortality was extremely low in small litters of primiparous females, potentially preventing us from finding an effect, offspring mortality was slightly higher in the larger litters of multiparous females. Increased maternal effort of mothers being born large relative to their siblings offset negative effects of their larger litter sizes, thereby increasing survival chances of their offspring. Usually, smaller pups in larger litters generally suffer from an increased early mortality risk which is elevated under unfavourable environmental conditions such as low temperatures (Trillmich et al. 2019). Similar effects have been found in the altricial European rabbit (Rödel et al. 2009b). While heavy born females had larger litters compared to their smaller sisters, their own offspring did not suffer from increased mortality but instead had significantly higher survival chances.
In rabbits, elevated mortality risk of smaller pups has been thoroughly investigated and is known to be caused by a combination of enhanced competitive abilities of larger littermates which enables them to gain a larger share of the mother’s milk (Bautista et al. 2005), increased thermoregulatory needs of smaller pups because they occupy peripheral positions in the litter huddle (Bautista et al. 2008) and elevated endoparasite load of smaller siblings (Rödel et al. 2020). In guinea pigs (Cavia porcellus), offspring of larger litters have to wait longer for access to teats, compete for access more and spend less time suckling, meanwhile eating more solid food (Fey and Trillmich 2008). Here, we found that maternal effort expended on offspring is higher in mothers being born large compared to their littermates both, before and after birth. While heavy born females produced on average larger litters, their offspring also grew more until weaning, which in turn lowered their mortality risk.
In summary, our study provides striking evidence that the early within-family environment exerts robust, long-term fitness consequences throughout life. While many studies have described short-term effects of early-life conditions on growth and survival of the individual, only few studies have investigated long-term fitness consequences and these might still be largely underestimated (but see Rödel et al. 2009a).
In addition to the effects of birth mass and size rank in relation to littermates on the timing of maturation and later reproductive output, the sex composition of a female´s litter had strong and robust effects on the growth of offspring in both, primi- and multiparous females and in addition on maternal effort in multiparous females. Offspring of mothers which grew up with brothers, had higher growth rates and maternal effort of multiparous females was likewise increased when they had grown up with brothers. Female embryos developing in proximity to male embryos experience increased androgen exposure and can become masculinised (Ryan and Vandenbergh 2002). Masculinisation, which is well reflected morphologically in the ano-genital distance, has been shown to affect various phenotypic characteristics such as reproductive traits, sex-ratio, physiology and morphology (Szenczi et al. 2013; Correa et al. 2021). Several studies report effects on the onset of reproduction. Studies on European rabbits and yellow-bellied marmots for example showed a delayed maturation for females growing up in male-biased litters but reported no effect on reproductive characteristics after maturation (Monclús et al. 2014). A study on female grey mouse lemurs (Microcebus murinus), reported a negative effect of having a brother on successful pregnancies during the first breeding season (Perret 2019). We here see more fine-scaled effects of the presence of brothers on maturation in which brothers only delay maturation in intermediate sisters but not in the largest sisters. At the same time however, we also see a delayed maturation in smallest females of all-female litters which might reflect competition among same-sexed pups for maternal resources. Alternatively, or in addition, mothers might provide more resources such as milk or care to male pups, irrespective of their size rank. Such male preference might explain a later onset of maturation (i.e., delayed development) and later lowered maternal effort of medium-sized females in mixed-sex litters while such an effect is not seen in all-female litters. However, while it is known that male pups are born on average ∼ 10% heavier and grow faster to weaning (Trillmich et al. 2019), no study yet investigated potential sex-biased differences in maternal care.
Comparable to our results on offspring growth, Correa et al. (2016) report for degus (Octodon degus), heavier offspring for masculinised females, i.e., females which likely grew up in male-biased litters. Similar to this and other studies before, we also found a male-biased sex-ratio in litters produced by females growing up with brothers (Ryan and Vandenbergh 2002; Correa 2012, 2016). In degus, higher offspring mass at weaning from masculinised females was caused by increased maternal care (Correa 2012) which has been suggested to increase offspring survival and fitness under harsh environmental conditions with increased competition (Correa et al. 2016). In contrast, studies on non-human primates found no to little effects of mixed-sex litters on reproductive characteristics (Bradley et al. 2016; French et al. 2016; McCoy et al. 2019) emphasising the differences in mammalian life-history strategies.
Finally, we also found effects of the maternal age, measured as the consecutive number of the litter in multiparous females. With consecutive litters, maternal effort increased while offspring growth to weaning decreased slightly. This resulted in a weak increase in juvenile mortality. In addition, litters became more male-biased with consecutive litters. Previous studies in the same species showed an increase of maternal effort and maternal condition until the age of about two years (Trillmich et al. 2019). Thus, the females used in this study were all still in the increasing phase of their reproductive output, well before the onset of senescence. All our females started their six months breeding cycle in spring, thus, we cannot fully exclude additional seasonal effects on maternal investment into reproduction. Litters born in summer months are generally larger in the number of pups than litters born in spring or autumn (Rübensam et al. 2015; AG et al., unpubl. data). In domesticated rabbits, the maternal effort increases up to the 4th to 6th parturition before starting to decline (Apori et al. 2014), similar to what we observe in cavies here and in previous studies (Trillmich et al. 2019). In contrast to the negative effects on offspring survival we report here, however, survival to maturity in wild rabbits increases with increasing maternal age (Rödel et al. 2009a). The increased offspring mortality and slightly lower offspring growth to weaning we observe, indicate that female cavies transfer the costs of reproduction to offspring as has been described for larger mammals (Clutton-Brock et al. 1996; Gaillard and Yoccoz 2003). Such an effect has already been described in the domesticated guinea pig when females have to reproduce under unfavourable temperatures (Trillmich and Guenther 2023).
Taken together, our results demonstrate that factors of the early family environment have long-term fitness consequences for the individual. Such individual effects might contribute to population-level dynamics (Sutherland 1996) and might mediate life-history, physiological and behavioural characteristics in populations of small mammals experiencing regular differences in density and therefore competitive regimes.
Data availability
The raw data associated with this article have been uploaded to the data server of Bielefeld University and is publicly available under https://doi.org/10.4119/unibi/2987968.
References
Apori SO, Hagan JK, Osei D (2014) The growth and reproductive performance of different breeds of rabbits kept under warm and humid environments in Ghana. Online J Anim Feed Res 5:51–59
Auguie B, Antonov A, Auguie MB (2017) Package ‘gridExtra’. Miscellaneous Functions for Grid Graphics, https://cran.r-project.org/web/packages/gridExtra/index.html
Bartoń K (2023) MuMIn: Multi-Model Inference. R package version 1.47.5, https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv:1406.5823
Bautista A, Mendoza-Degante M, Coureaud G, Martínez-Gómez M, Hudson R (2005) Scramble competition in newborn domestic rabbits for an unusually restricted milk supply. Anim Behav 70:1011–1021. https://doi.org/10.1016/j.anbehav.2005.01.015
Bautista A, García-Torres E, Martínez-Gómez M, Hudson R (2008) Do newborn domestic rabbits Oryctolagus cuniculus compete for thermally advantageous positions in the litter huddle? Behav Ecol Sociobiol 62:331–339. https://doi.org/10.1007/s00265-007-0420-4
Bradley BJ, Snowdon CT, McGrew WC, Lawler RR, Guevara EE, McIntosh A, O’Connor T (2016) Non-human primates avoid the detrimental effects of prenatal androgen exposure in mixed-sex litters: combined demographic, behavioral, and genetic analyses. Am J Primatol 78:1304–1315. https://doi.org/10.1002/ajp.22583
Caswell H, Hastings A (1980) Fecundity, developmental time, and population growth rate: an analytical solution. Theor Popul Biol 17:71–79. https://doi.org/10.1016/0040-5809(80)90015-5
Clutton-Brock TH, Stevenson IR, Marrow P, MacColl AD, Houston AI, McNamara JM (1996) Population fluctuations, reproductive costs and life-history tactics in female soay sheep. J Anim Ecol 65:675–689
R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/
Correa LA (2012) Mecanismos de regulacion del tama ∼ no de camada y razon de sexos en Octodon degus: efectos de la alostasis prenatal en la variacion fenotıpica de las crıas y sus consecuencias en la estabilidad de los grupos sociales. PhD thesis, Universidad Austral de Chile, Valdivia, Chile
Correa LA, Leon C, Ramírez-Estrada J, Soto‐Gamboa M, Sepulveda RD, Ebensperger LA (2016) Masculinized females produce heavier offspring in a group living rodent. J Anim Ecol 85:1552–1562. https://doi.org/10.1111/1365-2656.12588
Correa LA, León C, Ramírez-Estrada J, Ly-Prieto Á, Abades S, Hayes LD, Soto-Gamboa M, Ebensperger LA (2021) One for all and all for one: phenotype assortment and reproductive success in masculinized females. Behav Ecol 32:1266–1275. https://doi.org/10.1093/beheco/arab093
Darwin C (1859) On the origin of species by means of natural selection or the preservation of favored races in the struggle for life. John Murray, London
Dobson FS, Oli MK (2001) The demographic basis of population regulation in columbian ground squirrels. Am Nat 158:236–247. https://doi.org/10.1086/321322
Drummond H (2006) Dominance in vertebrate broods and litters. Q Rev Biol 81:3–32. https://doi.org/10.1086/503922
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton, NJ
Fey K, Trillmich F (2008) Sibling competition in guinea pigs (Cavia aperea f. porcellus): scrambling for mother’s teats is stressful. Behav Ecol Sociobiol 62:321–329. https://doi.org/10.1007/s00265-007-0419-x
Finkemeier MA, Trillmich F, Guenther A (2016) Match–mismatch experiments using photoperiod expose developmental plasticity of personality traits. Ethology 122:80–93. https://doi.org/10.1111/eth.12448
French JA, Frye B, Cavanaugh J, Ren D, Mustoe AC, Rapaport L, Mickelberg J (2016) Gene changes may minimize masculinizing and defeminizing influences of exposure to male cotwins in female callitrichine primates. Biol Sex Differ 7:28. https://doi.org/10.1186/s13293-016-0081-y
Gaillard JM, Yoccoz NG (2003) Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84:3294–3306. https://doi.org/10.1890/02-0409
Guenther A, Trillmich F (2013) Photoperiod influences the behavioral and physiological phenotype during ontogeny. Behav Ecol 24:402–411. https://doi.org/10.1093/beheco/ars177
Guenther A, Trillmich F (2015) Within-litter differences in personality and physiology relate to size differences among siblings in cavies. Physiol Behav 145:22–28. https://doi.org/10.1016/jphysbeh.2015.03.026
Guenther A, Trillmich F (2023) Photoperiod influences the development and the expression of personality traits and social behaviour in wild cavies (Cavia aperea). Ethology 129:33–46. https://doi.org/10.1111/eth.13343
Guenther A, Palme R, Dersen M, Kaiser S, Trillmich F (2014a) Photoperiodic effects on reproductive development in male cavies (Cavia aperea). Physiol Behav 123:142–147. https://doi.org/10.1016/j.physbeh.2013.10.017
Guenther A, Finkemeier MA, Trillmich F (2014b) The ontogeny of personality in the wild guinea pig. Anim Behav 90:131–139. https://doi.org/10.1016/j.anbehav.2014.01032
Guenther A, Eweleit L, Trillmich F (2021) Fitness consequences of seasonally different life histories? A match–mismatch experiment. Behav Ecol 32:500–507. https://doi.org/10.1093/beheco/araa149
Hudson R, Trillmich F (2008) Sibling competition and cooperation in mammals: challenges, developments and prospects. Behav Ecol Sociobiol 62:299–307. https://doi.org/10.1007/s00265-007-0417-z
Koskela E (1998) Offspring growth, survival and reproductive success in the bank Vole: a litter size manipulation experiment. Oecologia 115:379–384. https://doi.org/10.1007/s004420050531
Kraus C, Trillmich F, Künkele J (2005) Reproduction and growth in a precocial small mammal, Cavia magna. J Mammal 86:763–772. https://doi.org/10.1644/1545-1542(2005)086[0763:RAGIAP]2.0.CO;2
Kraus S, Trillmich F, Guenther A (2020) Within-family environment and cross-fostering stress affect behaviour and physiology in wild cavies (Cavia aperea). Front Psychol 11:178. https://doi.org/10.3389/fpsyg.2020.00178
Künkele J, Trillmich F (1997) Are precocial young cheaper? Lactation energetics in the guinea pig. Physiol Zool 70:589–596. https://doi.org/10.1086/515863
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Mazerolle MJ (2023) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3.2, https://cran.r-project.org/package=AICcmodavg
McCoy DE, Frye BM, Kotler J et al (2019) A comparative study of litter size and sex composition in a large dataset of callitrichine monkeys. Am J Primatol 81:e23038. https://doi.org/10.1016/j.isci.2021.103724
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, Oxford, UK
Monclús R, von Holst D, Blumstein DT, Rödel HG (2014) Long-term effects of litter sex ratio on female reproduction in two iteroparous mammals. Funct Ecol 28:954–962. https://doi.org/10.1111/1365-2435.12231
Nitsch A, Faurie C, Lummaa V (2013) Are elder siblings helpers or competitors? Antagonistic fitness effects of sibling interactions in humans. Proc R Soc B 280:20122313. https://doi.org/10.1098/rspb.2012.2313
Oli MK, Dobson FS (2003) The relative importance of life-history variables to population growth rate in mammals: Cole’s prediction revisited. Am Nat 161:422–440. https://doi.org/10.1086/367591
Perret M (2019) Litter sex composition affects first reproduction in female grey mouse Lemurs (Microcebus murinus). Physiol Behav 208:112575. https://doi.org/10.1016/j.physbeh.2019.112575
Raffel M, Trillmich F, Houner A (1996) Energy allocation in reproducing and non-reproducing guinea pig (Cavia porcellus) females and young under ad libitum conditions. J Zool 239:437–452. https://doi.org/10.4119/unibi/2979295
Rehling A, Trillmich F (2007) Weaning in the guinea pig (Cavia aperea f. porcellus): who decides and by what measure? Behav Ecol Sociobiol 62:149–157. https://doi.org/10.1007/s00265-007-0449-4
Rödel HG, von Holst D (2009) Features of the early juvenile development predict competitive performance in male European rabbits. Physiol Behav 97:495–502. https://doi.org/10.1016/j.physbeh.2009.04.005
Rödel HG, Bautista A, García-Torres E, Martínez-Gómez M, Hudson R (2008a) Why do heavy littermates grow better than lighter ones? A study in wild and domestic European rabbits. Physiol Behav 95:441–448. https://doi.org/10.1016/j.physbeh.2008.07.011
Rödel HG, Starkloff A, Bruchner B, von Holst D (2008b) Social environment and reproduction in female European rabbits (Oryctolagus cuniculus): benefits of the presence of litter sisters. J Comp Psychol 122:73. https://doi.org/10.1037/0735-7036.122.1.73
Rödel HG, von Holst D, Kraus C (2009a) Family legacies: short-and long-term fitness consequences of early-life conditions in female European rabbits. J Anim Ecol 78:789–797. https://doi.org/10.1111/j.1365-2656.2009.01537.x
Rödel HG, Starkloff A, Seltmann MW, Prager G, von Holst D (2009b) Causes and predictors of nest mortality in a European rabbit population. Mamm Biol 74:198–209. https://doi.org/10.1016/j.mambio.2008.04.003
Rödel HG, Oppelt C, Starkloff A, Prager N, Long E, Rüdiger A-T, Seltmann MW, Monclús R, Hudson R, Poteaux C (2020) Within-litter covariance of allele-specific MHC heterozygosity, coccidian endoparasite load and growth is modulated by sibling differences in starting mass. Oecologia 194:345–357. https://doi.org/10.14814/phy2.15427
Rood JP (1972) Ecological and behavioural comparisons of three genera of Argentine cavies. Anim Behav Monogr 5:1–83. https://doi.org/10.1016/S0066-1856(72)80002-5
Rood JP, Weir BJ (1970) Reproduction in female wild guinea-pigs. Reproduction 23:393–409. https://doi.org/10.1530/jrf.0.0230393
Rübensam K, Hribal R, Jewgenow K, Guenther A (2015) Seasonally different reproductive investment in a medium-sized rodent (Cavia aperea). Theriogenology 84:639–644. https://doi.org/10.1016/j.theriogenology.2015.04.023
Ryan BC, Vandenbergh JG (2002) Intrauterine position effects. Neurosci Biobehav Rev 26:665–678. https://doi.org/10.1016/S0149-7634(02)00038-6
Saal FV (1989) Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci 67:1824–1840. https://doi.org/10.2527/jas1989.6771824x
Sutherland WJ (1996) From individual Behaviour to Population Ecology. Oxford University Press, Oxford, UK. https://doi.org/10.1093/oso/9780198549116.001.0001
Szenczi P, Bánszegi O, Groó Z, Altbäcker V (2013) Anogenital distance and condition as predictors of litter sex ratio in two mouse species: a study of the house mouse (Mus musculus) and mound-building mouse (Mus spicilegus). PLoS ONE 8:e74066. https://doi.org/10.1371/journal.pone.0074066
Touma C, Palme R, Sachser N (2001) Different types of oestrous cycle in two closely related south American rodents (Cavia aperea and Galea musteloides) with different social and mating systems. Reproduction 121:791–801. https://doi.org/10.1530/rep.0.1210791
Trillmich F, Guenther A (2023) Shifts in energy allocation and reproduction in response to temperature in a small precocial mammal. BMC Zool 8:23. https://doi.org/10.1186/s40850-023-00185-6
Trillmich F, Mueller B, Kaiser S, Krause J (2009) Puberty in female cavies (Cavia aperea) is affected by photoperiod and social conditions. Physiol Behav 96:476–480. https://doi.org/10.1016/j.physbeh.2008.11.014
Trillmich F, Geißler E, Guenther A (2019) Senescence and costs of reproduction in the life history of a small precocial species. Ecol Evol 9:7069–7079. https://doi.org/10.5061/dryad.5k1b239
White PA (2002) Early cub mortality in the spotted hyena, Crocuta crocuta: effects of maternal rank, communal den use, and maternal favoritism. PhD thesis, University of California, Berkeley
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-, New York
Ylönen H, Horne TJ, Luukkonen M (2004) Effect of birth and weaning mass on growth. Evol Ecol Res 6:433–442
Zepeda JA, Rödel HG, Monclús R, Hudson R, Bautista A (2019) Sibling differences in litter huddle position contribute to overall variation in weaning mass in a small mammal. Behav Ecol Sociobiol 73:165. https://doi.org/10.1007/s00265-019-2777-6
Acknowledgements
We thank the caretakers of the Animal Behaviour department for taking good care of the animals. Furthermore, we greatly appreciate the many constructive comments by two anonymous reviewers.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG) as part of the research group “FOR 1232, TR105/22-1-2”. We acknowledge the Open Access Publication Fund of Bielefeld University for the article processing charge.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AG and FT designed the experiment. AG collected the data. SK analysed the data. SK and AG wrote the manuscript. All authors provided improvements to the manuscript and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval
All procedures reported here were taken during regular breeding events of the animals and thus do not need a specific ethics permission according to national guidelines. Housing facilities are regularly approved for keeping and breeding cavies by the local government authority responsible for health, veterinary and food monitoring (Gesundheits-, Veterinär- und Lebensmittelüberwachungsamt) under the licence number 530.42 16 30 − 1. All animals were used for other experiments or kept under stock conditions after the end of observations. The animals that died before the end of the study, died of diverse natural causes. Some juveniles were stillborn (early mortality < 5%). Three primiparous females died during or shortly after giving birth, and two males died due to kidney failure when about 6 months old.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. G Ophir.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kraus, S., Trillmich, F. & Guenther, A. Long-term effects of litter characteristics on reproduction in female cavies (Cavia aperea). Behav Ecol Sociobiol 78, 91 (2024). https://doi.org/10.1007/s00265-024-03508-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03508-w