Abstract
Insurance-based mechanisms, where surviving group-members can complete parental care after the death of a nestmate, are key to the origin of cooperative group formation in insects. Selection for group living via these models is proposed to be dependent on the life expectancy of adult carers relative to the duration of offspring dependency on parental care. Progressive provisioning, where adults feed offspring gradually as they grow, is thought to extend this period of dependency and is therefore suggested to be an important factor promoting the evolution of sociality. In contrast, mass-provisioning species provide offspring with all the food they need to reach maturity at the beginning of their development. Since offspring are then nutritionally independent, the applicability of insurance models is less clear. In this paper we experimentally demonstrate that adult presence on the nest, even after the end of provisioning, is critical for brood survival in the mass provisioning silk wasp Microstigmus rosae. After 10 days, experimentally orphaned nests contained 65% fewer healthy offspring than controls. Adult females were also recorded performing post-provisioning parental care behaviours including nest maintenance and repair, putative hygienic brood care and aggressive nest defence against both ants and parasitoid wasps. By demonstrating the potential applicability of insurance advantages our results highlight how, even in mass provisioners, insurance-based mechanisms may be part of what favours group living.
Significance statement
Extended parental care is an important precursor to the evolution of eusociality. In this context, group living can serve as a form of “life insurance”, ensuring that dependent offspring receive the care they need to reach maturity should the mother die. Such mechanisms are especially important to our understanding of social evolution as they are able to account for the origins of cooperative group formation, not just its maintenance. However, for mass-provisioning species, where all food items are provided upfront, the significance of insurance advantages remains unclear. In this study, we experimentally demonstrate that adult attendance is critical for brood survival in the mass provisioning wasp, Microstigmus rosae. Our results reveal the applicability of insurance advantages to M. rosae with important implications for our understanding of the potential adaptive value of group living in mass provisioning species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While parental care is relatively rare in insects, many aculeate Hymenoptera (the stinging bees, wasps, and ants) invest substantial effort in caring for their young, provisioning them with food items and protecting them from enemies, often until maturation (Córdoba-Aguilar et al. 2018; Marshall 2023). Such sustained contact between parents and offspring, or “extended parental care”, is thought to provide the ideal circumstances for the evolution of group living (Lin and Michener 1972; Queller 1994; Socias-Martínez and Kappeler 2019). However, prolonged dependency on parental care leaves offspring at risk; if the parent dies, immature offspring will not reach adulthood (Eickwort et al. 1996; Kukuk et al. 1998; Smith et al. 2003). One means of ensuring against this risk is for adult relatives to form social groups. Then, if one carer dies others remain to care for developing offspring. In this way, cooperative group formation may function as a kind of “life insurance”, ensuring that brood receive the extended care they need to reach maturity (Queller 1994; Field et al. 2000; Shreeves et al. 2003). Insurance advantages, also termed “assured fitness returns”, are important to our understanding of social evolution as, although many theories are put forward to explain the maintenance of social traits, far fewer are able to account for their origins (Schwarz et al. 2010).
Insurance models suggest that group living will be selected for in species where the lifespan of adults is unlikely to be long enough for parental care requirements to be fulfilled by one individual alone. Thus, selection for group living will be dependent on the length of adult life expectancy relative to the duration of offspring dependency on parental care (Strassmann et al. 1989; Gadagkar 1990, 1991; Queller 1994). Within Hymenoptera, this level of dependency can vary substantially according to the way that parents provide food to their offspring. Food provisioning usually involves one of two alternative strategies: progressive or mass provisioning. In progressively provisioning species, adults provide larvae with food items gradually throughout their development, so that offspring are always entirely dependent on the presence of a provisioning adult (Schwarz 1988; Field 2005). Here the risks associated with solitary living are clear: if a lone female dies, any offspring she has not yet finished provisioning will starve and her parental investment will be lost. In contrast, mass provisioned offspring are provided with all the food they need at the beginning of their development. Cells are then sealed, and it is typically assumed that no further offspring-parent interactions take place. In mass provisioners, offspring are therefore nutritionally independent early on in their development, effectively reducing the risk of brood failure should the mother die, and potentially diminishing selection for helping behaviour via assured fitness returns (Field 2005).
To the extent that it increases the period of offspring dependency, progressive provisioning is thought to facilitate the evolution of cooperation and has even been proposed as a necessary precursor for the evolution of eusociality (Wheeler 1928; Michener 1969; Hunt 1999; Wilson 2008; Nowak et al. 2010). However, food provisioning is not the only form in which parental care occurs. Parental care, broadly defined as “any parental trait that enhances the fitness of a parent’s offspring, and that is likely to have originated and/or to be currently maintained for this function” (Royle et al. 2012), incorporates a range of important behaviours such as the building and maintenance of nesting structures, hygienic brood care and defence from parasites and predators (Field 1992; Mappes and Kaitala 1994; Nalepa and Bell 1997; Kukuk et al. 1998; Smith et al. 2003; Field and Brace 2004; Maekawa et al. 2008; Miller et al. 2011; Boos et al. 2014; Quiñones and Wcislo 2015; Peterson et al. 2016). While food provisioning undeniably constitutes a significant component of parental care in many insects, this broader spectrum of parental care behaviours may also be important in their potential to confer insurance advantages for social group formation.
These ideas are most easily appreciated by considering Field’s (2005) models which compare the reproductive success of mass- and progressive provisioning strategies under two alternative parental care scenarios. The first model is constructed around the assumption that immature offspring become independent as soon as they are fully provisioned. Here, simultaneous progressive provisioning (where several offspring are provisioned progressively at the same time) prolongs the period of offspring dependency and thus increases the risk that a mother will die before her offspring reach independence. This model depicts the assumptions made in early discussions of insurance-based mechanisms and represents the conditions under which progressive provisioning would be expected to favour group living (Gadagkar 1990; Queller 1994). Field’s second model considers an alternative scenario whereby offspring are dependent on parental care not only for food provisioning but also for protection against predators and parasites. Under these conditions, the period of offspring dependency differs less, or not at all, between progressive and mass provisioning. In the latter case, insurance-based advantages to potential helpers will be independent of provisioning strategy, and neither is expected to favour the evolution of helping through differences in offspring dependency alone (Field 2005).
Using a combination of observational data and experimental manipulations, we here examine the extent of offspring dependency in the incipiently social Microstigmus rosae (Field 2023). Most M. rosae nests contain only one adult female, but around 20% are home to groups of up to four cohabiting females exhibiting high reproductive skew (Bonifacii and Field 2023). Adult females fully mass-provision each brood cell before laying the egg, provisioning one offspring at a time and remaining on the nest to provide for subsequent offspring. As an early-stage social, M. rosae has the potential to be a valuable system to examine the evolutionary ecology of group living (Bonifacii and Field 2023). As discussed above, insurance-based models offer potential advantages to group living contingent on the assumption of offspring dependency on extended parental care. By experimentally examining the importance of parental care behaviour for offspring survival, we here test this assumption in the mass provisioning M. rosae.
Methods
Overview
To investigate whether the presence of adults on the nest directly impacts offspring survival in the mass provisioning M. rosae, we conducted an adult-removal experiment. To do this, we permanently removed adult females from 14 experimental nests, while in 15 control nests, females were removed and immediately released nearby. We collected nests after a 10-day interval and analysed the effect of our experimental treatment on the number and condition of brood using generalised mixed-effects models. We further examined the nature of parental care exhibited by M. rosae through observation of naturally occurring behaviour collected from video recordings of 14 additional nests. These nests were distinct from those included as treatment or controls in the main experiment. To assess whether adults of M. rosae exhibit defensive behaviour against ant intruders, a significant threat in tropical environments, we experimentally introduced ants to ten M. rosae nests and recorded subsequent adult behaviour. To explore possibility of chemical repellent on the nest petiole, we made half of these introductions directly onto the nesting substrate (leaf) rather directly than onto the nest itself.
Adult removal experiment
This research was carried out between 14th January and 10th April 2017 in Mashpi Biodiversity Reserve, located in the Pichincha province of North-western Ecuador (N 00°10.019' W 078°52.326'). Within this reserve, M. rosae is common and often builds nests on its preferred host plant, Xanthosoma sagittifolium, along with the less numerous M. mirandae and M. lydiae (Field 2023). We examined whether adult presence provides a direct benefit for offspring survival in M. rosae by conducting a female-removal experiment. The enclosed nest structure of this species means that it is not possible to monitor brood development and survival in real time (Fig. 1). Therefore, to ensure that experimental and control group nests contained a similar range and number of brood, we conducted our experiment on nests of known age. To obtain nests of known founding date, we tagged and systematically monitored 956 Xanthosoma sagittifolium plants along an 8km trail which spans the reserve. Every 5 days, each nesting location was examined for both the appearance of newly founded nests and the continued presence of existing nests. Upon discovery, we assigned each nest a unique identification code and the location and date of its appearance was recorded. This method enabled us to assign the date of nest founding to a five-day window. In M. rosae, nests are founded by a single female (Bonifacii and Field 2023). Thus, by using newly established nests and collecting them before the first offspring emerge, we were able to control for any potential group size effects by including only single female nests in our experiment.
Our experimental design was informed by the offspring developmental data presented in Bonifacii & Field (2023) and was as follows (Fig. 2): 27 days after nest discovery, we permanently removed the single adult female from half of the identified nests (experimental group, n = 14 nests). The remaining half served as controls (n = 15). In this group, to control for any potential effects of nest manipulation, we removed adult females but immediately released them nearby. Experimental manipulations took place before sunrise to ensure that a) all nest occupants were inside the nest and b) that adults from control group nests were able to safely return during the day that followed. By assigning nests alternately to treatments as they were located, we attempted to match control and experimental group nests by altitude and date founded as far as possible.
Timeline for the adult removal experiment, including approximate stages of offspring development. The numbers along the second row indicate the days since nest founding (day 1), and subsequent rows symbolises the average developmental progression of successive offspring within the nest. The duration of foraging and offspring developmental stages presented are based on the average values for M. rosae presented in Bonifacii & Field (2023). The area shaded blue indicates the range of days within which nests were discovered (days 1–5), the orange area indicates when the experimental treatment took place (day 25–29), and the green area indicates when nests were collected (day 35–39). Hatched cells represent those offspring excluded from the analysis as they were produced in control nests after the experimental treatment took place
Day 27 was deliberately chosen for our experimental manipulation so as to ensure that a range of offspring developmental stages were present in the nests when adults were removed. Developmental data show that typical nests in this age range contain around 2–3 brood, the eldest of which is at least at the prepupa stage. As the detected parasites for this species are known to attack prepupae, ensuring nests were old enough to contain brood at this developmental stage was an important consideration (Bonifacii & Field 2023).
To remove adults, we placed small re-sealable plastic bags around the nests and, taking care not to cause any damage, gently compressed the nests through these bags. The disturbance causes the occupants to leave the nest and become trapped in the re-sealable bags. For experimental nests, we confirmed that the species was M. rosae using a Leica S6D binocular microscope before placing adults in 100% ethanol. Adults from the control group were species checked using a field lens, and IDs were later verified using a Leica microscope.
To gain a reliable measure of the potential payoff to parental investment, it was important to make sure that offspring did not mature and leave the nest before the end of the experiment. Brood development takes a minimum of 45 days in M. rosae (Bonifacii and Field 2023). We therefore chose to collect nests ten days after the experimental manipulation, when they were between 37and 41 days old. All nests were carefully collected by detaching the nest petiole from the substrate and placing them into resealable bags. Collections took place in the evening to ensure that all nest occupants were inside the nest. The same evening, we dissected nests using the Leica microscope and recorded their contents. Care was also taken to check for the presence of several distinct signs of nest degradation: the occurrence of parasitism (including the number, size and developmental stage of any parasites), dead and decaying brood, nests without any offspring (likely the result of ant predation) and clear signs of structural damage. We also recorded any occurrences of entire nest disappearance. After dissection, all nest contents were stored in 100% ethanol for subsequent genetic analysis.
After experimental removals, adults in control group nests had an additional ten days in which to produce offspring before nest collection. As a result, the productivity of these nests will be inherently higher than in that of experimental group nests. To correct for this, we excluded brood at the egg or larval stages from productivity calculations. According to developmental data, 13 days is needed for an egg to reach the pre-pupal stage of development. Thus, any eggs laid in control nests after female removal on experimental group nests will be in the larval stage ten days later. By omitting offspring in the larval or egg stages, we are thereby able to eliminate any unfair productivity advantages accrued in control group nests.
Adult behaviour
To further investigate the nature of parental care provided by adult M. rosae we collected and examined video footage of naturally occurring adult behaviour on the nest. Observational data were collected using Sony HDR-PJ330 camcorders mounted on tripods and directed towards the nest entrance so that the entire nest was visible on the recorded footage. Nests were filmed for 1–3 h each day, depending on weather conditions. On occasions where the weather prevented filming for more than two hours on any one nest, we made up the time on a subsequent day. Altogether we filmed 14 different nests over 8 days between 19th February 2017 and 26th March 2017.
Ant introductions
To investigate the existence of defensive behaviour against ants, we made ten experimental ant introductions to M. rosae nests between 11 and 16th March 2017. Five of these introductions were intended to test for the possibility of chemical repellent on the nest petiole (Jeanne 1970; Kojima 1992). To do this we used masking tape to create a square on the underside of the leaf forming a border around the petiole of each nest of approximately 5cm x 5cm. The tape was then coated in a thin layer of TangleFoot insect barrier to prevent the introduced ants from escaping. Using forceps, a single small ant was introduced onto the leaf on the inside of this boundary and its behaviour observed. For the remaining five nests, the same methodology was used but ants were placed directly onto nests. The ants used for these introductions were taken from the same source colony and were therefore assumed to be from the same, unidentified, species. All introductions were filmed using Sony HDR-PJ330 camcorders mounted on tripods.
Statistical analyses and linear models
All statistical analyses were performed using R version 4.3.0 (http://www.r-project.org) (R Core Team 2023) and RStudio Version 2023.03.1 + 446 (https://www.rstudio.com) (RStudio Team 2020). Models were performed using the package ‘glmmTMB’ (version 1.1.7; Brooks et al. 2017) a flexible package which allows for the implementation of generalized linear mixed effect models with a wide range of error structures. The effect of the experimental treatment on the total number of healthy brood found in nests was tested using a “compois”, Conway-Maxwell Poisson, error structure, with date-founded and altitude included as random effects. This error structure was chosen by fitting all suitable alternatives for count data and selecting the model giving the smallest AIC.
To examine the likelihood of signs of nest and brood deterioration (1/0), we implemented a logistical regression using a binomial error structures. We conducted multiple statistical tests, each aimed at examining offspring dependency on distinct components of parental care behaviour: “empty nest”, “dead/ mouldy brood”, “structural damage” and “parasitism”. A final test, “any deterioration,” provided a comprehensive analysis using all available data. With the exception of “structural damage”, the indicators examined in these tests are mutually exclusive, so that a brood identified with one form of deterioration (e.g., predation) could not simultaneously exhibit another form (e.g., parasitism or mouldiness). Given the independence of these tests and their focus on unique aspects of parental care, we use a significance level of p = 0.05 for these analyses. Model checks were performed using the ‘DHARMa’ package (version 0.4.6; Hartig 2022).
Results
Adult removal experiment
We used a total of 29 nests for the experimental analysis, 14 experimental group nests and 15 controls. One experimental group nest disappeared during the experiment. As nest disappearance is likely to be the result of predation or extreme weather conditions rather than parental behaviour, this nest was excluded from the analyses. In addition, one control group nest was found with the adult dead inside the nest upon collection. As there is no way to determine at which point this individual died, and thus the extent of parental care that was received by the offspring, we also omitted this nest. Lastly, three of the experimental group nests had one or more adults present when collected at the end of the experiment- most likely resulting from the appropriation of nesting structures by conspecific females. Brood adoption is probably a natural outcome for orphaned nests, and the following results include these three data points. However, analyses conducted both with and without these nests and results were qualitatively equivalent. Statistical analysis reveals no significant difference between experimental and control group nests in either altitude (p = 0.87) or the date founded (p = 0.34).
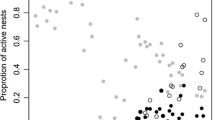
There was a highly significant effect of female removal on the total number of healthy brood found within nests upon collection (Fig. 3, LRT1 = 10.19, p = 0.001, GLMM: Family = “compois”, n = 27). Control group nests were found to contain an average of 1.78 healthy brood (± SD 0.97), contrasting with experimental group nests where less than one surviving offspring was present on average (0.62 ± 0.77). These values represent a 65.2% reduction in the number of surviving brood as a direct consequence of female removal. Justifying the exclusion of younger offspring from the productivity calculations, one experimental group nest was found containing a larva, compared with six control group nests. The remaining experimental nests contained no offspring of an earlier developmental stage than prepupae. The fact that larvae were observed in an experimental nest demonstrates that the chosen offspring exclusions were, if anything, over-cautious, potentially increasing the chances of a false negative result.
Overall, experimentally orphaned nests were significantly more likely to show at least one clear indication of nest deterioration (parasitism/ dead offspring/ structural damage or a complete absence of brood) than control group nests (LRT1 = 8.8, p < 0.003, GLMM, Family = Binomial, n = 27). Of the examined indicators, orphaned nests were significantly more likely to have a total absence of brood (LRT1 = 5.42, p = 0.02), to contain dead and decaying brood (LRT1 = 4.8, p = 0.03) and for the nest to show signs of structural damage (LRT1 = 4.8, p = 0.03). There was no difference in the incidence of parasitism between the two groups (LRT1 = 0.094, p = 0.76). The total number of nests exhibiting each indictor, separated by experimental treatment, are presented in Table 1. Three nests from the experimental group showed none of the listed indicators of nest deterioration. However, when compared with control group nests, these nests still had a marginally significantly lower number of brood (LRT1 = 3.78, p = 0.052), possibly indicating partial brood predation.
Maternal behaviour
As observed on video recordings, adult M. rosae females, were seen performing several parental care behaviours including offspring provisioning, nest maintenance and repair, and aggressive nest defence against parasitoid wasps. Females made regular departures from the nest, often returning with visible prey or nesting materials held within their mandibles. Nest maintenance behaviours were also commonly recorded and included inspection walks around the nest and apparent cleaning and repair. Cleaning took place both inside and outside the nest and wasps were often observed removing particles, held in the mandibles, by standing on the outside of the nest and raising the anterior part of the body before dropping them off the nest. Although we were unable to determine the exact source of the particles being removed in this manner, they may be faecal remains or fragments of rejected nest material. Notably, no removals of brood or parasites from the nest were observed, and parasitised and dead pupae were found within collected nests upon dissection. In addition to “housekeeping” behaviour, females were observed spending significant amounts of time moving slowly around the nest and petiole performing distinctive abdominal movements likely associated with the application of silk to the nest surface. Our video recordings also showed several incidences of nests being visited by parasitiod wasps, although we did not directly witness any oviposition taking place. On one occasion a nest was visited by a very small chalcidid wasp which was quickly chased away by the resident wasp. On several other occasions, larger parasitoid wasps were seen visiting nests, but no defensive behaviour was observed.
Ant introductions
In four of the five nests where ants were placed directly onto the nesting substrate, mothers were seen to exhibit defensive behaviour towards the introduced individual, including aggressive attacking both from the nest and from the air whilst flying. In the fifth nest, the resident wasp may have been absent at the time of the introduction as no adult female was observed. Aggressive responses continued until ants either left nests via the petiole or were displaced from the nesting structure. Ants placed on the substrate did not appear to be able to enter the nest via the petiole. However, three of the five ants introduced onto the nesting structure were seen to leave via the petiole, often after several attempts. These observations may indicate that any chemical repellent is restricted to the base of the petiole, where the petiole meets the nesting substrate. One of the nests contained an adult male which immediately flew from the nest when the ant was introduced.
Discussion
While progressive provisioning is the dominant strategy among eusocial lineages, social behaviour also occurs in mass provisioning groups including sweat bees (Danforth and Eickwort 1997), carpenter bees (Xylocopinae) (Sless and Rehan 2023), the only eusocial apoid wasps Microstigmus (Matthews 1968; Bonifacii and Field 2023) and the highly eusocial stingless bees (Meliponidae) (Herre and Wcislo 2011). Nevertheless, almost all the empirical studies of insurance advantages have examined progressively provisioned species. Examples include the tropical hover wasp Liostenogaster flavolineata (Field et al. 2000), the paper wasp Polistes dominula (Shreeves et al. 2003) and the silk wasp Microstigmus nigrophthalmus (Lucas and Field 2011). These studies have successfully established the validity of insurance-based models for the evolution of helping behaviour in progressively provisioning species. In contrast, for many mass provisioning species, it remains unclear whether insurance-based models are applicable, as the consequences of female mortality on offspring survival are less clear (Gadagkar 1990).
Through an adult removal experiment, we here demonstrate that adult presence on the nest, even after the end of provisioning, is critical for brood survival in the mass provisioning M. rosae. Ten days after experimental orphaning, nests contained 65% fewer healthy offspring than control nests. We have additionally documented adult M. rosae females performing several post-provisioning parental care behaviours including nest maintenance and repair, putative hygienic brood care and aggressive nest defence against both ants and parasitoid wasps. By demonstrating that M. rosae offspring continue to rely on extended parental care, even after provisioning is complete, our results validate the applicability of insurance advantages to this mass provisioning, incipiently social species. Together with studies of mass provisioning sweat bees where adult presence also significantly decreases the incidence of brood mortality (Eickwort et al. 1996; Kukuk et al. 1998; Smith et al. 2003), this suggests that the link between progressive provisioning and insurance-based advantages may be less concrete than historically implied. Under these conditions, the period of offspring dependency will differ less, or not at all, between progressive and mass provisioning species. In the latter case, insurance-based advantages to potential helpers will be independent of provisioning strategy, and neither would be expected to favour the evolution of helping through differences in offspring dependency alone (Field 2005).
Extending these insurance-based models beyond progressive provisioners offers the chance to focus on the role of other forms of parental care in the evolution of sociality. Our results also lend support to the idea that extrinsic factors such as the prevalence of predators and parasites can be major selective forces favouring the evolution of group living (Strassmann et al. 1989; Crespi 1994; Queller and Strassmann 1998). That said, in contrast with Field & Brace’s (2004) findings for the non-social apoid wasp Ammophila, we find no evidence to suggest that adult presence on the nest is effective at preventing offspring parasitism in M. rosae. Given that our video footage revealed active nest defence against parasites, these results are surprising. However, with high rates of entire brood predation in experimental group nests (38.5%), the available nests for which parasitism could be detected was just 7/13. Adjusting for this, the rate of parasitism in orphaned nests is calculated to be 28%, compared to 14.2% in control nests. Moreover, the assumption that empty nests were solely predated may overlook the potential occurrence of prior parasitism. Considering these limitations, it seems plausible that our experiment might not fully capture the true rate of parasitism in orphaned nests.
A further explanation could be that the lone females we studied, which must leave the nest undefended while foraging, are unable to provide effective protection against parasitism. This idea is corroborated by Bonifacii & Field’s (2023) data, where almost all instances of interspecific parasitism were found to befall single female M. rosae nests. If groups of females can provide better protection for developing brood, the resulting increase in brood survival may be an important selection pressure in the creation and maintenance of social groups.
Our observations of an increased number of mouldy and decaying brood in experimentally orphaned nests may contradict the common assumption that mass provisioning precludes hygienic brood care by adults (Field and Brace 2004). These results, in conjunction with the lack of faecal matter found inside cells containing immature M. rosae brood (Bonifacii and Field 2023), suggests that adult females are able to open brood cells to inspect and clean developing brood. Behaviour which has been demonstrated in several other mass-provisioning species (Batra and Bohart 1969; Plateaux-Quénu 2008; Rehan et al. 2009; Rehan and Richards 2010; Quiñones and Wcislo 2015). Such hygienic brood maintenance may be more common than appreciated, and particularly relevant to the evolution of social group formation. Increased social contact inherent to group living significantly increases the risk of exposure to pathogens and parasites (Fefferman et al. 2007). By attenuating this increased risk of disease, hygienic behaviour may function to reduce costs associated with social group formation and may therefore represent a significant preadaptation for social life (Plateaux-Quénu 2008).
The conditions found in M. rosae, whereby offspring risk mortality if the mother dies after provisioning is complete but before offspring reach adulthood, are closest to the conditions represented by the second model in Field’s (2005) paper. However, there is one important difference between the life history of M. rosae and that considered by Field’s models. Field’s models were based on the life history of the best-studied social wasps (vespoids), in which both mass and progressive provisioners lay eggs in the empty cell before provisioning commences. In apoid wasps and bees, while progressive provisioners lay the egg at the start of provisioning, most mass provisioners oviposit only once provisioning is complete and a full provision mass has been collected. Although it appears slight, this difference has significant implications. In such “forage first” mass provisioners, the time between a mother starting to forage and offspring maturing is effectively increased by the time it takes to provision each cell (a process which takes up to 8 days in M. rosae (Bonifacii and Field 2023)). Therefore, the mother must survive longer to see each offspring through to independence. As a result, in forage first taxa, offspring dependency on extended parental care could theoretically lead to stronger selection for group living under mass- than under progressive provisioning. Any association between progressive provisioning and sociality would then occur for other reasons, such as the demographic advantage that progressive provisioning can provide (Field 2005).
Conclusion
Our study emphasises the complex and multifaceted nature of parental care. Offspring reliance on parental care, and thus the duration of offspring dependency, is likely to be highly contingent on the specific biology and behaviours of the species in question, which must be examined before making assumptions about the consequences of care for the evolution of sociality. By demonstrating the potential applicability of insurance advantages our results highlight how, even in mass provisioners, insurance-based mechanisms may be part of what favours group living. However, well-supported demonstrations of insurance-based mechanisms favouring the evolution of helping behaviour in mass-provisioning species are lacking. As the potential applicability of these mechanisms has now been established, M. rosae represents a good prospective study system with which to further explore theoretical predictions.
Data availability
The datasets generated during and/or analysed during the current study are available in the figshare repository, https://figshare.com/articles/dataset/_/24175122.
References
Batra SW, Bohart GE (1969) Alkali bees: response of adults to pathogenic fungi in brood cells. Science 165:607. https://doi.org/10.1126/science.165.3893.607
Bonifacii RL, Field J (2023) Nesting biology and social organisation of a silk wasp (Microstigmus rosae) from the North-West Ecuadorian Choco. Insectes Soc 70:167–179. https://doi.org/10.1007/s00040-023-00914-7
Boos S, Meunier J, Pichon S, Kölliker M (2014) Maternal care provides antifungal protection to eggs in the European earwig. Behav Ecol 25:754–761. https://doi.org/10.1093/beheco/aru046
Brooks ME, Kristensen K, van Benthem KJ, et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. Rj 9:378–400. https://doi.org/10.32614/RJ-2017-066
Córdoba-Aguilar A, González-Tokman D, González-Santoyo I (eds) (2018) Insect behavior: from mechanisms to ecological and evolutionary consequences. Oxford University Press. https://doi.org/10.1093/oso/9780198797500.001.0001
Crespi BJ (1994) Three conditions for the evolution of eusociality: are they sufficient? Insectes Soc 41:395–400. https://doi.org/10.1007/BF01240642
Danforth BN, Eickwort GC (1997) The evolution of social behavior in the augochlorine sweat bees based on a phylogenetic analysis of the genera. The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press, Cambridge, pp 270–315
Eickwort GC, Eickwort JM, Gordon J et al (1996) Solitary behavior in a high-altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Behav Ecol Sociobiol 38:227–233. https://doi.org/10.1007/s002650050236
Fefferman NH, Traniello JFA, Rosengaus RB, Calleri DV (2007) Disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior, and colony organization. Behav Ecol Sociobiol 61:565–577. https://doi.org/10.1007/s00265-006-0285-y
Field J (1992) Intraspecific parasitism and nest defence in the solitary pompilid wasp Anoplius viaticus (Hymenoptera: Pompilidae). J Zool 228:341–350. https://doi.org/10.1111/j.1469-7998.1992.tb04613.x
Field J (2005) The evolution of progressive provisioning. Behav Ecol 16:770–778
Field J (2023) Description and nesting biology of three new species of neotropical silk wasp (Hymenoptera: Apoidea: Pemphredoninae: Microstigmus). J Nat Hist 57:1–18. https://doi.org/10.1080/00222933.2022.2157345
Field J, Brace S (2004) Pre-social benefits of extended parental care. Nature 428:650–652. https://doi.org/10.1038/nature02427
Field J, Shreeves G, Sumner S, Casiraghi M (2000) Insurance-based advantage to helpers in a tropical hover wasp. Nature 404:869–871. https://doi.org/10.1038/35009097
Gadagkar R (1990) Evolution of eusociality: the advantage of assured fitness returns. Philos Trans Biol Sci 329:17–25. https://doi.org/10.1098/rstb.1990.0146
Gadagkar R (1991) Demographic predisposition to the evolution of eusociality: a hierarchy of models. Proc Natl Acad Sci U S A 88:10993–10997
Hartig F (2022) DHARMa: Residual Diagnostics for hierarchical (multi-level / mixed) regression models. R Package Version 046:5
Herre EA, Wcislo WT (2011) In defence of inclusive fitness theory. Nature 471:E8–E9. https://doi.org/10.1038/nature09835
Hunt JH (1999) Trait mapping and salience in the evolution of eusocial vespid wasps. Evolution 53:225–237. https://doi.org/10.2307/2640935
Jeanne RL (1970) Chemical Defense of Brood by a Social Wasp. Science 168:1465–1466. https://doi.org/10.1126/science.168.3938.1465
Kojima JI (1992) The ant repellent function of the rubbing substance in an Old World polistine, Parapolybia indica (Hymenoptera Vespidae). Ethol Ecol Evol 4:183–185. https://doi.org/10.1080/08927014.1992.9525339
Kukuk PF, Ward SA, Jozwiak A (1998) Mutualistic benefits generate an unequal distribution of risky activities among unrelated group members. Naturwissenschaften 85:445–449. https://doi.org/10.1007/s001140050528
Lin N, Michener CD (1972) Evolution of Sociality in Insects. Q Rev Biol 47:131–159. https://doi.org/10.1086/407216
Lucas ER, Field J (2011) Assured fitness returns in a social wasp with no worker caste. Proc R Soc B Biol Sci 278:2991–2995. https://doi.org/10.1098/rspb.2011.0128
Maekawa K, Matsumoto T, Nalepa CA (2008) Social biology of the wood-feeding cockroach genus Salganea (Dictyoptera, Blaberidae, Panesthiinae): did ovoviviparity prevent the evolution of eusociality in the lineage? Insectes Soc 55:107–114. https://doi.org/10.1007/s00040-008-0997-2
Mappes J, Kaitala A (1994) Experiments with Elasmucha grisea L. (Heteroptera: Acanthosomatidae): does a female parent bug lay as many eggs as she can defend? Behav Ecol 5:314–317. https://doi.org/10.1093/beheco/5.3.314
Marshall SA (2023) Hymenoptera: The natural history and diversity of wasps, bees and ants. Firefly Books Ltd, Buffalo
Matthews RW (1968) Microstigmus comes: sociality in a sphecid wasp. Science 160:787–788. https://doi.org/10.1126/science.160.3829.787
Michener CD (1969) Comparative social behavior of bees. Annu Rev Entomol 14:299–342. https://doi.org/10.1146/annurev.en.14.010169.001503
Miller JS, Rudolph L, Zink AG (2011) Maternal nest defense reduces egg cannibalism by conspecific females in the maritime earwig Anisolabis maritima. Behav Ecol Sociobiol 65:1873–1879. https://doi.org/10.1007/s00265-011-1196-0
Nalepa CA, Bell WJ (1997) Postovulation parental investment and parental care in cockroaches. In: The evolution of social behavior in insects and arachnids. Cambridge University Press Cambridge, pp 26–51
Nowak MA, Tarnita CE, Wilson EO (2010) The evolution of eusociality. Nature 466:1057–1062. https://doi.org/10.1038/nature09205
Peterson JH, Hoffmeister TS, Roitberg BD (2016) Variation in maternal solitary bee nest defence related to nest state. Apidologie 47:90–100. https://doi.org/10.1007/s13592-015-0378-6
Plateaux-Quénu C (2008) Subsociality in halictine bees. Insectes Sociaux 55:335–346. https://doi.org/10.1007/s00040-008-1028-z
Queller DC (1994) Extended parental care and the origin of eusociality. Proc Biol Sci 256:105–111
Queller DC, Strassmann JE (1998) Kin selection and social insects. Bioscience 48:165–175. https://doi.org/10.2307/1313262
Quiñones AE, Wcislo WT (2015) Cryptic extended brood care in the facultatively eusocial sweat bee Megalopta genalis. Insectes Soc 62:307–313. https://doi.org/10.1007/s00040-015-0409-3
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 30 Jan 2024
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Rehan SM, Richards MH (2010) Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Can Entomol 142:65–74. https://doi.org/10.4039/n09-056
Rehan SM, Richards MH, Schwarz MP (2009) Evidence of social nesting in the Ceratina of Borneo (Hymenoptera: Apidae). J Kans Entomol Soc 82:194–209. https://doi.org/10.2317/JKES809.22.1
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of parental care. Oxford University Press
Schwarz MP (1988) Local resource enhancement and sex ratios in a primitively social bee. Nature 331:346–348
Schwarz MP, Tierney SM, Rehan SM et al (2010) The evolution of eusociality in allodapine bees: workers began by waiting. Biol Lett 7:277–280. https://doi.org/10.1098/rsbl.2010.0757
Shreeves G, Cant MA, Bolton A, Field J (2003) Insurance–based advantages for subordinate co–foundresses in a temperate paper wasp. Proc R Soc Lond B Biol Sci 270:1617–1622. https://doi.org/10.1098/rspb.2003.2409
Sless T, Rehan S (2023) Phylogeny of the carpenter bees (Apidae: Xylocopinae) highlights repeated evolution of sociality. Biol Lett 19:20230252. https://doi.org/10.1098/rsbl.2023.0252
Smith AR, Wcislo WT, O’Donnell S (2003) Assured fitness returns favor sociality in a mass-provisioning sweat bee, Megalopta genalis (Hymenoptera: Halictidae). Behav Ecol Sociobiol 54:14–21. https://doi.org/10.1007/s00265-003-0589-0
Socias-Martínez L, Kappeler PM (2019) Catalyzing transitions to sociality: ecology builds on parental care. Front Ecol Evol 7:160. https://doi.org/10.3389/fevo.2019.00160
Strassmann JE, Queller DC (1989) Ecological determinants of social evolution. In: The Genetics Of Social Evolution. CRC Press, pp 81–101
Wheeler WM (1928) The social insects: their origin and evolution. Routledge
Wilson EO (2008) One giant leap: how insects achieved altruism and colonial life. Bioscience 58:17–25. https://doi.org/10.1641/B580106
Acknowledgements
Specimens were collected under the “autorizaciones de investigacion cientifica: No 004-14 IC-FAU-FLO-DNB/MA, No 008-15 -IC-FAU-DNB/MA and No 009- 2017- IC- FAU-DPAP- MA.” Thank you to Anna and Carmen Cambugan at Fundación Cambugan for help in obtaining the necessary research permits, to Santiago Villamarin at the MECN Quito for cooperation with this process and to the Ministerio del Ambiente, Ecuador for granting these permissions. Special thanks to Dr Mika Peck for introducing us to the field sites and helping set up the research project and to our field assistants Brinna Ellen Louisa Barlow, Charlie Dance and Jamie Wallis for the invaluable assistance provided in the field.
Funding
This research was funded by the University of Sussex and the University of Exeter.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by R.B. The first draft of the manuscript was written by R.B. with comments and editing by J.F. J.F. supervised the project. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: W. Hughes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonifacii, R.L., Field, J. Extended parental care in the mass provisioning silk wasp, Microstigmus rosae. Behav Ecol Sociobiol 78, 20 (2024). https://doi.org/10.1007/s00265-024-03437-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03437-8