Abstract
Pheromones are perhaps the most common form of intraspecific communication in the animal kingdom and used in various contexts. Their modulatory potential on cognitive processes has been demonstrated in both vertebrates and invertebrates. Particularly interesting in this regard are social insects, due to their extensive use of pheromones to organise collective behaviour. Recruitment pheromones might be expected to encourage learning, but could also hinder learning due to a blocking effect, whereby the pheromone already partially predicts the reward, hindering further cues being associated with the reward. Here, we use free-running learning assays using realistic pheromone strength to test for a modulation effect on learning in the black garden ant Lasius niger. We found no evidence that learning in three modalities (olfactory, visual, and spatial) is affected by the presence of a realistic pheromone trail. Interestingly, this is in contrast to findings in honeybees. The fact that associative learning does not seem to be influenced by recruitment pheromone in L. niger and reportedly the Argentine ant, while it is in honeybees, the possibly best-studied social insect species, is noteworthy. We speculate that a species-specific importance of social information use could drive modulatory effects of pheromones on a wide range of cognitive processes.
Significance statement
Pheromones have been shown to modulate associative learning in a variety of animals. Among social insects, attractive pheromone has been found to enhance associative olfactory learning in honeybees but not in ants. In ants, recruitment pheromone predicts a food source; therefore, it might hinder learning of a new cue for a food reward. We use a free-running learning assay to test for an effect of trail pheromone on associative learning in three different modalities—olfactory, spatial, visual—in Lasius niger, but find no evidence of any effect. Our learning assay demonstrated fast olfactory learning, moderate spatial learning, and no visual learning after only one training visit. Based on our findings, and findings in two other ant species, we speculate that the ecological foraging conditions of mass-recruiting ants, i.e. following a trail, have not favoured a modulation potential of recruitment pheromone opposed to attractive pheromone in honeybees.
Similar content being viewed by others
Introduction
Pheromones are used in various contexts across the animal kingdom (Wyatt 2017), such as territory marking (Hediger 1949; Hölldobler and Wilson 1977), mating (Hill et al. 1979, p. 197; Gaskett 2007; Stowers and Liberles 2016), oviposition (Seenivasagan and Vijayaraghavan 2010), feeding (Schaal et al. 2003), alarm (Urlacher et al. 2010; Wang et al. 2016), and recruitment (Crawford and Rissing 1983; Aron et al. 1993; Czaczkes and Ratnieks 2012; Hoefele et al. 2020). Over the past two decades, research on the modulatory potential of pheromones on learning processes was brought into focus. Pheromones have been shown to enhance or inhibit associative learning in a variety of animals such as rabbits (Schaal et al. 2003), rats (Carew et al. 2018), moths (Murmu et al. 2020), honeybees (Vergoz et al. 2007; Urlacher et al. 2010; Baracchi et al. 2020), and ants (Rossi et al. 2020).
Particularly interesting are the modulatory effects of pheromones in insects, since insect communication relies heavily on pheromones, especially among social insects (Wyatt 2017). The study of insect cognition offers insight into the underlying mechanisms of cognitive processes in arguably simpler and more tractable models (Menzel et al. 2006; Dornhaus and Franks 2008; Giurfa 2015; Czaczkes 2022). Furthermore, insect pheromones and their components are well studied, thus enabling targeted hypothesis testing (Murmu et al. 2020). In the honeybee Apis mellifera, for example, Urlacher et al. (2010) showed that the sting alarm pheromone impaired olfactory learning. The underlying mechanism implicated in this impairment was changes in motivation induced by the pheromone when exposed prior to conditioning. Baracchi et al. (2020) showed that motivational changes, induced by aversive or attractive pheromone components, were mediated via aminergic signalling and can impair or enhance learning and memory (Baracchi et al. 2020). While there is a tendency to generalise from honeybees to other (social) insects, this is often not warranted: The Argentine ant, Linepithema humile, when pre-exposed to a trail pheromone component only showed an increased sucrose acceptance, while olfactory learning and memory were unaffected (Rossi et al. 2020). This implies that pheromones may modulate the perceived valence of a reward but not necessarily impact associative learning.
In the above-mentioned studies, both species, honeybees and Argentine ants, were pre-exposed to pheromone components prior to conditioning, but did not experience the pheromones concurrently with an unconditional stimulus. While pre-exposure might be biologically meaningful in some occasions (e.g. the use of alarm pheromone (Jeanne 1981)), in central place foraging ants, individuals are often recruited to food sources using trail pheromones (Hölldobler and Wilson 1990; Detrain et al. 1999; Cassill 2003; Evison et al. 2008). Recruited individuals thereby experience both trail pheromone and stimuli associated with the food reward simultaneously.
Using a free-running set up, the modulatory effects of trail pheromone on sucrose acceptance and olfactory learning were tested over two studies in the black garden ant, Lasius niger. In the first study on sucrose acceptance, Oberhauser et al. (2020) let individual ants walk on a runway leading to a drop of sucrose, with either trail pheromone or a solvent control on the runway. On reaching the sucrose, they tested whether food acceptance changed depending on pheromone presence. The ants showed no effect of pheromone on their sucrose acceptance rate, even when bitter quinine was added to the sucrose to avoid ceiling effects. In a second study Oberhauser et al. (2022) tested whether trail pheromone affected olfactory learning in this species. The ants experienced a rewarded odour on a runway either simultaneously with trail pheromone or with a solvent control. No difference in learning between control and pheromone treatment was found. These results are in contrast to the previous findings in honeybees and partially to Argentine ants, leading to the question of whether L. niger is unaffected by pheromone modulation or whether it could not be detected due to ceiling effects in learning rates.
However, presenting two conditional stimuli (CS) simultaneously during associative learning can also lead to cue competition, i.e. reduced association of one or both of the stimuli to the unconditional stimulus (US), or no association of one as predicted by the concepts of overshadowing and blocking (Mackintosh 1971). Both concepts explain how the simultaneous presentation of two conditional stimuli results in one stimulus having less or even no predictive power associated with a reward (US). The difference between overshadowing and blocking is that overshadowing is observed when one of the two conditional stimuli has a stronger predictive power even though both stimuli were neutral to the individual prior to associative learning (Pavlov 1927; Mackintosh 1976; Schubert et al. 2015), while blocking is based on one stimulus having a preestablished associative relationship to the US thus blocking the other stimulus from forming an association to the US (Kamin 1968). Previously, overshadowing has been attributed to the salience of the conditioned stimuli (Pavlov 1927; Mackintosh 1976); however, more recent work on honeybees provided evidence that the cumulative experience and the generalisation profiles of a stimulus shapes the predictive power instead (Schubert et al. 2015).
We propose that trail pheromone could exert a similar suppressive effect on associative learning. This assumes that pheromone is innately predictive of the presence of food (US). Indeed, Ito and Hojo (2022) have shown that under certain conditions, pheromone presence can reduce associative learning in a free-running assay on ants. In honeybees, blocking by queen pheromone (Vergoz et al. 2007) or preconditioned olfactory or visual cues has been shown as well as in other species (Blaser et al. 2006; Guez and Miller 2008; Acebes et al. 2009).
Lasius niger has been shown to learn new associations between salient stimuli such as odour cues and food rewards within one training visit (Czaczkes and Kumar 2020; Oberhauser et al. 2022). Even in spatial learning assays using a T-maze ants have achieved learning rates of 65–81% after only one visit (Grüter et al. 2011; Czaczkes et al. 2015a; Oberhauser et al. 2018). Such fast learning rates might result in potential ceiling effects and hence could mask any positive effect of pheromone on learning. Testing stimuli with slower learning rates could uncover positive modulatory effects of pheromones on learning, while faster learning rates allow the investigation of negative effects.
Aside from the learning concepts, the relative importance of trail pheromones in navigation might also influence whether these pheromones modulate learning: Although L. niger has been shown to prefer individual memory over pheromone as social information (Grüter et al. 2011), these ants are unable to learn to avoid trail pheromones, but they can learn to ignore them (Wenig et al. 2021). This implies that pheromones have a strong, but not overwhelming, impact on decision making in this species. Li. humile, for example, is much more strongly influenced by trail pheromones (Aron et al. 1993; von Thienen et al. 2014). In order to examine modulatory effects on learning—positive or negative—the learned associations need to be weak enough to avoid ceiling effects but strong enough to observe learning. Testing other modalities with less salient stimuli might help disentangle potentially masked effects of pheromone on learning in Lasius niger.
Thus, the aim of this study was to assess the impact of trail pheromone on associative learning success in L. niger while avoiding ceiling effects. We performed a series of three experiments each testing a different modal stimulus—olfactory learning, spatial learning and visual learning.
Material and methods
Study species
We used twelve queenless Lasius niger colony fragments (henceforth colonies) consisting of ~ 1000 workers. Colonies were collected on the campus of the University of Regensburg from twelve wild colonies and housed in plastic foraging boxes (32.5 cm × 22.2 cm × 11.4 cm) with a plaster of Paris floor and PTFE-coated walls. Each box contained a circular plaster nest (ø 14 cm and 2 cm height) and were kept at a 12:12 light–dark cycle at room temperature (21–26 °C) with access to water ad libitum. Between experiments, ants had ad libitum access to 0.5 M sucrose solution and were fed three times per week with cockroaches. During experiments, ants were deprived of sucrose solution 4 days prior to testing to ensure high motivation.
Pheromone extraction
Artificial pheromone of realistic strength was produced by dissecting eight worker hindgut glands and immersing them in 2 ml of dichloromethane (DCM), following von Thienen et al. (2014). The effectiveness of the artificial pheromone was then tested on a Y-maze, where 5 µl pheromone were applied in a straight line on one arm. Pheromone following rate of two colonies was assessed for each arm of the Y-maze. Artificial pheromone was deemed suitable for use when a following rate of around 70–85% was reached, which corresponds to the pheromone following rate for naturally-laid trails (von Thienen et al. 2014).
Setup and experimental procedure
General
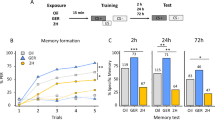
We conducted three experiments, each testing associative learning in one modality. The experiments consisted of one trial each. During a trial, an ant had to undergo one training visit and one test visit, i.e. a total of two visits. We chose one training visit since previous experiments have resulted in reliable learning rates of 65–81% after only one training visit (Grüter et al. 2011; Oberhauser et al. 2018; Czaczkes and Kumar 2020). A trial started with a training visit in which an individual ant was allowed to enter either a runway (20 cm long and 1 cm wide) or a Y-maze (arms and stem 10 cm long, 1 cm wide, tapering to 2 mm at the bifurcation, see Fig. 1) via a drawbridge, depending on the modality (see below). Drawbridge, runway, and Y-maze were covered with disposable paper overlays on which either a trail of pheromone as treatment or dichloromethane (DCM) as a solvent control was applied. In the case of the Y-maze, solution or solvent was only applied on the stem. In the training visit, ants were presented with a stimulus which was rewarded with a 1 M sucrose droplet. After finding the sucrose, the ant was allowed to return to the nest to unload her sucrose load to her nestmates. During this time, the test apparatus was prepared. Training details for each modality are described below. The learning success of each ant was then tested in the following visit. In the test, the ant could freely choose between the two arms of a Y-maze, one of which would present the trained stimulus while the other would not. The side of the Y-maze offering the reward-associated cue was systematically varied between ants. Since the Y-maze does not restrict the ants to only one choice, we considered the crossing of a decision line 2 cm past the bifurcation point as the ‘initial choice’ and the crossing of a decision line 8 cm past the bifurcation point as the ‘final choice’. After testing, ants were removed from the colony in order to avoid pseudo-replication.
Experimental procedure. Each ant underwent one trial (consisting of a training and a test visit) in one of the presented stimulus modalities—olfactory, visual, or spatial. The respective stimuli were rewarded with 1 mol l−1 of sucrose solution in the training visit. Rewarded cues: For olfactory learning, ants were trained on lemon scented paper overlays. Only one stimulus was used, since the salience of the stimulus has been shown to be sufficient in previous studies (Oberhauser et al. 2022). For visual learning, ants were trained on either of the two contrasts (light or dark grey, hex codes: #AFABAB and #3B3838) to improve salience of the cues during testing. Contrasts were chosen to exclude colour preference and to ensure comparability to other species if needed. Each contrast was printed on paper and presented on walls surrounding the runway. For spatial learning, ants were trained to either side of the Y-maze, and to balance bias towards a side, each ant was rewarded on the respective side it did not visit first. Training (1st visit): Ants were freely allowed to forage on the setup; the first ant to enter was then trained. Ants were trained on either a straight 20-cm-long runway (olfactory and visual modalities) or on a Y-maze with a 10-cm-long stem leading to a bifurcation with two 10-cm-long arms each in a 60° angle (spatial modality). Pheromone or dichloromethane (DCM) was applied on the paper overlays, either 10 µl on the 20-cm runway or 5 µl on the 10-cm stem of the Y-maze arm. A drop of 1 mol l−1 sucrose solution was placed on the end of each paper overlay. After an ant was allowed to drink until fulfilment during which she was marked with a dot of acrylic paint on the gaster, the ant returned to the nest to unload her load to the nestmates. Test (2nd visit): Ants were tested on a Y-maze, the same as used for spatial training. The trained stimulus was presented on one side of the maze while nothing (olfactory learning) or the other was presented on the other side. No reward was given and ants were permanently removed from the colony after testing
Olfactory learning
Training: The runway was covered with a lemon scented paper overlay. Paper overlays were scented by storing them in an airtight plastic box containing an open glass Petri dish with 0.5 mL lemon food flavouring (www.flavorline.de) for at least 12 h. Pheromone and DCM was balanced across ants. On the overlay, either 10 µL pheromone or DCM was freshly applied in a straight line using a glass microcapillary (Servopax GmbH, Germany). A drop of 1 M sucrose solution was placed at the end of the runway on the overlay. In contrast to the visual and spatial stimulus, ants were trained to only one scent, as we knew from previous studies this is sufficient to form a very robust memory (Czaczkes and Kumar 2020).
Test: The linear runway was replaced by a Y-maze. All parts of the maze (stem and both arms) were covered with paper overlays. One arm (balanced across ants) was covered with a lemon scented paper overlay, while the others were unscented.
Visual learning
Training: A runway was covered with unscented paper overlay and surrounded by a 3D printed wall (23.4 cm × 4.4 cm × 4.8 cm (length × width × height) and 2 mm thick). The wall was fully covered with either light or dark grey printed paper (hex codes: #AFABAB and #3B3838, respectively). We used these contrasts to prevent potential biases due to colour or satiation preference during testing, to ensure stimulus intensity, and for comparability of the visual stimuli. Training of each contrast was balanced across ants, i.e. each ant was trained to one contrast. Either 10 µL of pheromone or of DCM was freshly applied on the paper overlay of the runway. Pheromone and DCM was balanced across ants.
Test: The linear runway and the surrounding wall was replaced by a Y-maze which was completely surrounded by a 3D printed wall (each arm and stem was 10.8 cm long forming 3.6 cm wide corridors with 0.2 cm thick walls). The Y-maze was covered with paper overlays. The inside of the surrounding wall was covered with printed paper so that one side of the maze was light grey and the other side dark grey (see Fig. 1). Contrast sides were balanced across ants.
Spatial learning
Training: A Y-maze was covered with unscented paper overlays. On the overlay of the stem and both arms either 5 µL of pheromone or of DCM was freshly applied. Ants were allowed to freely explore the Y-maze. The rewarded and thus trained side was always the one which was opposite to the first explored side of the ant. This ensures that the ants were trained against any innate side bias which they might have had. Sides and treatment were balanced across ants.
Test: Paper overlays of the Y-maze were replaced with fresh ones and the ant could freely choose between the sides.
Statistical analysis
The effects of pheromone and stimulus modality on learning success were analysed in R v.3.6.3 (http://www.r-project.org/) using a generalised linear mixed model (GLMM) (Bolker et al. 2009). GLMMs were fitted using the glmmTMB package (Brooks et al. 2017). We used the following logistic regression:
Final decision = treatment (pheromone/control) * stimulus (olfactory/spatial/visual) + random effect (colony ID, random intercept)
The independent variables, treatment and stimulus, were included as an interaction to account for potential effects of pheromone which might occur depending on the modality of the stimulus. The level of significance was set at P < 0.05. Model fit, dispersion and zero inflation were checked using the DHARMa package (Hartig and Lohse 2022). Model results were obtained using the ANOVA function of the car package (Fox et al. 2023). Post hoc tests were conducted with estimated marginal means from the emmeans package (Lenth et al. 2023). Figures were created using the ggplot2 package (Wickham et al. 2023) and the Effects package (Fox et al. 2022).
Data availability
The entire code and analysis output is provided in electronic supplementary material ESM1. The entire raw data on which this analysis is based is provided in electronic supplementary material ESM2.
Results
The proportion of ants choosing correctly was not affected by trail pheromone presence (χ2 = 0.32; P = 0.58), which was confirmed by post hoc tests (olfactory: odds ratio [OR] 0.58; P = 0.38; spatial: OR 1; P = 1; visual: OR 0.89; P = 0.73). However, the proportion of correct choices was significantly affected by the modality of the stimulus (χ2 = 22.97; P < 0.001, see Fig. 2). Post hoc analysis confirmed that this was the case for all stimulus types: Ants trained on an olfactory stimulus chose the correct arm significantly more often than would be expected by chance, with a 90% probability in the control (P < 0.001) and 84% probability in the pheromone group (P < 0.001). By contrast, ants trained on spatial stimuli in both control and pheromone treatment had a 61% probability of choosing correctly (P = 0.14 in each treatment group). In the visual modality, ants had a 61% probability of choosing correctly in the control treatment (P = 0.38) and a 55% probability in the pheromone treatment (P = 0.80). Given that we found no difference between treatment and control groups in any modality, we reanalysed the data leaving out treatment effect, to ask whether overall learning occurred. When considered this way, post hoc analysis showed that ants in the spatial modality showed evidence of learning with a probability of 61% (P = 0.037), while ants in the visual modality test still did not (55% probability, P = 0.38).
Proportion of correct decision. Ants trained on the olfactory stimulus chose 90% and 84% correctly in the test in the solvent control and pheromone treatment, which was significantly above chance (P < 0.001) but was not significantly different between both groups (P = 0.38). Ants trained on a spatial stimulus chose 61% correctly in both treatment groups, which was not significant from chance level (P = 0.14) and not significant between both groups (P = 1). Ants trained on a visual stimulus chose 61% and 55% correctly in the control and the pheromone treatment, which was not significant from chance (P = 0.8) and not significantly different between the groups (P = 0.73)
Discussion
In this study, we investigated a potential modulatory effect of trail pheromone on learning success in the ant Lasius niger using three different stimulus modalities. We found no effect of trail pheromone on learning success, supporting previous findings in the olfactory modality in this species (Oberhauser et al. 2020, 2022). Furthermore, our results demonstrated the impressive learning speed of L. niger when trained on an olfactory stimulus, with at least 84% of ants choosing correctly after a single training visit. These results are in line with other studies reporting rapid olfactory learning in this (Oberhauser et al. 2020; De Agrò et al. 2020; Czaczkes and Kumar 2020) and other ant species (Piqueret et al. 2019; Wagner et al. 2022). The spatial learning assay resulted in 61% of ants choosing correctly which was still significantly above chance when both control and treatment group were pooled together (see “Results” section). However, the visual learning assay resulted in 55% of ants choosing correctly, which was not different from chance, even when both control and treatment group where pooled. This was surprising, as both modalities can be learned by these ants after several training visits and show moderate reliability of side learning after the first visit to the correct arm (76%, Grüter et al. 2011; 70%, Oberhauser et al. 2019; 65–80% Oberhauser et al. 2018). Since data were collected by one person and the setup had been successfully used in previous studies, we cannot explain the low learning rate in those two modalities. For spatial learning, we can only speculate that the strict rule to reward the side visited second might have made it more difficult than in other experiments with pre-assigned rewarded sides.
Yet, this range of strong to very moderate learning is precisely what we were aiming for. Although the high olfactory learning rate might obscure any weak effect, strong effects—positive or negative—could still be detectable. Ants have been shown to improve learning rates over consecutive visits up to 100% (Oberhauser et al. 2018; De Agrò et al. 2020); thus, strong enhancement of olfactory learning could possibly still be observable, as well as of course strong inhibition. Neither was the case here. Our results of the spatial-learning assay further suggest that trail pheromone has no effect on learning in L. niger. As both treatment and control group exhibit the same chance-level response rates, which when pooled result in significant learning, this indicates that any modulation effect would be reflected here. A positive effect would likely show significant learning in the treatment group, while we would expect a negative effect to lower the pooled learning rate to a chance-level response rate. This assay provides the strongest evidence of a lack of a pheromone modulation effect, since both control and treatment group showed the same response rate. For visual learning, there was no clear learning to enhance nor block. Nonetheless, proving a negative is notoriously difficult, and we refrain from making strong statements here.
The presence of trail pheromone showed no evidence of modulating learning in either this study or in Oberhauser et al. (2022), and neither did it modulate sucrose acceptance in Oberhauser et al. (2020). This strongly suggests that neither reward valence nor learning is affected by trail pheromone presence in L. niger in a free-running set up. These results partially contrast previous findings in the Argentine ant Linepithema humile and in the honeybee Apis mellifera, in which attractive pheromone components increased reward valence, i.e. sucrose acceptance (Baracchi et al. 2017, 2020; Rossi et al. 2020). However, while an attractive pheromone component (geraniol) was found to improve learning in honeybees (Baracchi et al. 2020), learning was not affected by pre-exposure to a trail pheromone component in the Argentine ant (Rossi et al. 2020), mirroring the current results. Interestingly, results of another recent study report a negative effect of trail pheromone in high concentrations on learning in the clonal ant Pristomyrmex punctatus. Ito and Hojo (2022) tested the effect of trail pheromone following at varying concentrations on olfactory learning using a free-running set up almost identical to that used in our experiment. The results suggest attention-like processes based on intensity and order of stimulus to regulate between following behaviour and learning: The effect could be diminished by priming the ants with the respective odour stimulus (Ito and Hojo 2022). Note that further studies investigating pheromone modulation of cognitive processes used pheromones with functions other than recruitment, such as alarm (Urlacher et al. 2010), mating (Murmu et al. 2020), caste differentiation (Vergoz et al. 2007), and feeding (Schaal et al. 2003; Coureaud et al. 2006, 2010). Our findings only refer to pheromone used in a recruitment context. We cannot exclude that in L. niger, in another context, such as alarm, pheromones could also inhibit appetitive learning (Urlacher et al. 2010; Wang et al. 2016), facilitate aversive learning (Carew et al. 2018) or increase responsiveness to noxious stimuli (Rossi et al. 2018) as it was found in bees and rats.
Why does pheromone modulation of learning differ between eusocial insect species? Ecological conditions and constraints during foraging could be the culprits. While appetitive learning in three mass-recruiting ant species seems to be unaffected by trail pheromone (Rossi et al. 2020; Oberhauser et al. 2022), in honeybees, possibly the best-studied social insect (Menzel and Giurfa 2006; Menzel 2012), an attractive pheromone appears to upregulate signalling of biogenic amines, leading to enhanced appetitive learning (Baracchi et al. 2020). The potentially crucial difference between ants and bees during foraging is simple: ants walk and bees fly. This allows ants to follow a trail and use this trail during route learning while paying attention to other cues and integrating these during navigation (Czaczkes et al. 2013, 2016; De Agrò et al. 2020; Ito and Hojo 2022). Social bees such as honeybees, on the other hand, are recruited in the colony or at a food source where they are exposed to pheromones and where they may receive instructions, e.g. through waggle dances, on how to navigate to and from a food source (Wenner et al. 1969; Thom et al. 2007; Schürch and Grüter 2014). Foraging without the guidance of a pheromone trail could have therefore favoured the quick modulation of associative learning in the appropriate context.
It is also worth noting that biogenic amines involved in foraging activities and cognitive processes in both bees and ants (Barbero et al. 2023) seem to be regulated differentially. In honeybees, sucrose acceptance and appetitive learning are positively affected by an attractive pheromone component through increased biogenic amine signalling, namely of octopamine and dopamine (Baracchi et al. 2020). In ants, dopamine levels in foraging workers are higher than in other workers, suggesting that dopamine is involved in foraging activities (Seid and Traniello 2005; Cuvillier-Hot and Lenoir 2006). In L. niger, it was recently found that octopamine inhibition decreased appetitive olfactory learning, suggesting that octopamine is likely involved in the reward signalling pathway to the mushroom bodies. Dopamine inhibition, in the same study, led to interference of long-term memory formation (Wissink and Nehring 2021). Another recent study on Argentine ants, using very similar methods to those used in the current study, showed that the consumption of neither octopamine nor dopamine affected appetitive learning (Galante and Czaczkes 2022). Interestingly, Linn et al. (2020) showed that the consumption of octopamine decreased waggle dance following in honeybees compared to the control group and increased the use of private information, likely due to an increased perceived value of the food source caused by octopamine. In the same study, dopamine, expected to reduce perceived value, increased dance following in the treated bees, but it did not increase social information use. These results, although they did not specifically test learning in honeybees, contrast with those of Galante and Czaczkes (2022) on ants, further emphasising a differential modulation potential of these amines between the group of ants and bees, even though both groups seem to have the same underlying regulatory signalling pathways.
The modulatory effects of pheromone could be an adaptive function, reflecting the importance of associative learning in the presence of social information in a given ecological condition. Social information can be more important for some species than others, depending on their ecology (see Grüter and Leadbeater 2014). Especially when colonies can improve colony foraging success through efficient information transfer from scouts or scouting foragers to recruited nestmates (Schürch and Grüter 2014; Czaczkes et al. 2015b; Glaser et al. 2021; Shackleton et al. 2023). We speculate therefore that the absence of continuous social information such as trail pheromones during navigation could have favoured a fast modulation of cognitive processes when recruitment occurs, e.g. through signalling processes of biogenic amines involved in foraging as can be observed in the honeybee (Baracchi et al. 2020; Linn et al. 2020; Barbero et al. 2023). While honeybee foragers have been shown to prefer private over social information (Grüter et al. 2008; I’Anson Price et al. 2019; Linn et al. 2020), colony foraging success could still profit from modulatory effects of pheromone (Tait and Naug 2022). In ants, the constant guidance of trail pheromone during the entire foraging trip (Czaczkes et al. 2013, 2016; De Agrò et al. 2020) might mean that upregulating learning when in the presence of a trail is not beneficial. In other words, since bees fly and their recruitment pheromone is only present at the goal (a food source or the nest entrance) (Free and Williams 1972), it might make sense for the pheromone to upregulate learning. As ants walk along pheromone trails for the majority of their foraging cycle, upregulating learning over such a large area could be unhelpful.
In summary, our results provide no evidence that trail pheromone has an effect on learning of different stimulus modalities in L. niger. The free-running paradigm used here poses an ecologically realistic scenario of pairing stimulus and pheromone. Therefore, we conclude that even if trail pheromone would have weak undetected modulatory effects, they appear to have no ecological relevance for L. niger. However, it is important to note that other pheromones such as queen or alarm pheromones could affect the cognitive processes of L. niger differently and should be investigated in future studies. Furthermore, a comparative approach on studying modulatory effects of recruitment pheromone shows great potential of gaining insights into the evolution of communication in insect societies.
References
Acebes F, Solar P, Carnero S, Loy I (2009) Blocking of conditioning of tentacle lowering in the snail (Helix aspersa). Q J Exp Psychol 62:1315–1327. https://doi.org/10.1080/17470210802483545
Aron S, Beckers R, Deneubourg JL, Pasteels JM (1993) Memory and chemical communication in the orientation of two mass-recruiting ant species. Ins Soc 40:369–380. https://doi.org/10.1007/BF01253900
Baracchi D, Devaud J-M, d’Ettorre P, Giurfa M (2017) Pheromones modulate reward responsiveness and non-associative learning in honey bees. Sci Rep 7:9875. https://doi.org/10.1038/s41598-017-10113-7
Baracchi D, Cabirol A, Devaud J-M et al (2020) Pheromone components affect motivation and induce persistent modulation of associative learning and memory in honey bees. Commun Biol 3:1–9. https://doi.org/10.1038/s42003-020-01183-x
Barbero F, Mannino G, Casacci LP (2023) The role of biogenic amines in social insects: with a special focus on Ants. Insects 14:386. https://doi.org/10.3390/insects14040386
Blaser RE, Couvillon PA, Bitterman ME (2006) Blocking and pseudoblocking: new control experiments with honeybees. Q J Exp Psychol 59:68–76. https://doi.org/10.1080/17470210500242938
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Carew SJ, Mukherjee B, MacIntyre ITK et al (2018) Pheromone-induced odor associative fear learning in rats. Sci Rep 8:17701. https://doi.org/10.1038/s41598-018-36023-w
Cassill D (2003) Rules of supply and demand regulate recruitment to food in an ant society. Behav Ecol Sociobiol 54:441–450. https://doi.org/10.1007/s00265-003-0639-7
Coureaud G, Moncomble A-S, Montigny D et al (2006) A pheromone that rapidly promotes learning in the newborn. Curr Biol 16:1956–1961. https://doi.org/10.1016/j.cub.2006.08.030
Coureaud G, Charra R, Datiche F et al (2010) A pheromone to behave, a pheromone to learn: the rabbit mammary pheromone. J Comp Physiol A 196:779–790. https://doi.org/10.1007/s00359-010-0548-y
Crawford DL, Rissing SW (1983) Regulation of recruitment by individual scouts in Formica oreas Wheeler (Hymenoptera, Formicidae). Ins Soc 30:177–183. https://doi.org/10.1007/BF02223867
Cuvillier-Hot V, Lenoir A (2006) Biogenic amine levels, reproduction and social dominance in the queenless ant Streblognathus peetersi. Naturwissenschaften 93:149–153. https://doi.org/10.1007/s00114-006-0086-1
Czaczkes TJ, Kumar P (2020) Very rapid multi-odour discrimination learning in the ant Lasius niger. Insect Soc 67:541–545. https://doi.org/10.1007/s00040-020-00787-0
Czaczkes TJ, Ratnieks FLW (2012) Pheromone trails in the Brazilian ant Pheidole oxyops: extreme properties and dual recruitment action. Behav Ecol Sociobiol 66:1149–1156. https://doi.org/10.1007/s00265-012-1367-7
Czaczkes TJ, Grüter C, Ellis L et al (2013) Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. J Exp Biol 216:188–197. https://doi.org/10.1242/jeb.076570
Czaczkes TJ, Czaczkes B, Iglhaut C, Heinze J (2015) Composite collective decision-making. Proc R Soc B Biol Sci 282:20142723. https://doi.org/10.1098/rspb.2014.2723
Czaczkes TJ, Grüter C, Ratnieks FLW (2015) Trail pheromones: an integrative view of their role in social insect colony organization. Annu Rev Entomol 60:581–599. https://doi.org/10.1146/annurev-ento-010814-020627
Czaczkes TJ, Weichselgartner T, Bernadou A, Heinze J (2016) The effect of trail pheromone and path confinement on learning of complex routes in the ant Lasius niger. PLoS ONE 11:e0149720. https://doi.org/10.1371/journal.pone.0149720
Czaczkes TJ (2022) Advanced cognition in ants. Myrmecological News 32:
De Agrò M, Oberhauser FB, Loconsole M et al (2020) Multi-modal cue integration in the black garden ant. Anim Cogn 23:1119–1127. https://doi.org/10.1007/s10071-020-01360-9
Detrain C, Deneubourg J-L, Pasteels JM (1999) Decision-making in foraging by social insects. In: Detrain C, Deneubourg JL, Pasteels JM (eds) Information Processing in Social Insects. Birkhäuser Basel, Basel, pp 331–354
Dornhaus A, Franks NR (2008) Individual and collective cognition in ants and other insects (Hymenoptera: Formicidae). Myrmecological News 11:12
Evison SEF, Petchey OL, Beckerman AP, Ratnieks FLW (2008) Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behav Ecol Sociobiol 63:261. https://doi.org/10.1007/s00265-008-0657-6
Fox J, Weisberg S, Price B et al (2022) effects: Effect Displays for Linear, Generalized Linear, and Other Models
Fox J, Weisberg S, Price B et al (2023) car: Companion to Applied Regression
Free JB, Williams IH (1972) The role of the Nasonov gland pheromone in crop communication by honeybees (Apis mellifera L.). Behaviour 41:314–318
Galante H, Czaczkes TJ (2022) Invasive ant learning is not affected by seven potential neuroactive chemicals. 2022.11.01.514620
Gaskett AC (2007) Spider sex pheromones: emission, reception, structures, and functions. Biol Rev 82:27–48. https://doi.org/10.1111/j.1469-185X.2006.00002.x
Giurfa M (2015) Learning and cognition in insects. Wiley Interdiscip Rev Cogn Sci 6:383–395. https://doi.org/10.1002/wcs.1348
Glaser SM, Feitosa RM, Koch A et al (2021) Tandem communication improves ant foraging success in a highly competitive tropical habitat. Insect Soc 68:161–172. https://doi.org/10.1007/s00040-021-00810-y
Grüter C, Leadbeater E (2014) Insights from insects about adaptive social information use. Trends Ecol Evol 29:177–184. https://doi.org/10.1016/j.tree.2014.01.004
Grüter C, Balbuena MS, Farina WM (2008) Informational conflicts created by the waggle dance. Proc R Soc B Biol Sci 275:1321–1327. https://doi.org/10.1098/rspb.2008.0186
Grüter C, Czaczkes TJ, Ratnieks FLW (2011) Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav Ecol Sociobiol 65:141–148. https://doi.org/10.1007/s00265-010-1020-2
Guez D, Miller RR (2008) Blocking and pseudoblocking: the reply of Rattus norvegicus to Apis mellifera. Q J Exp Psychol 61:1186–1198. https://doi.org/10.1080/17470210701480238
Hartig F, Lohse L (2022) DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models
Hediger H (1949) Säugetier-Territorien und ihre Markierung. Bijdragen Tot De Dierkunde 28:172–184
Hill AS, Rings RW, Swier SR, Roelofs WL (1979) Sex pheromone of the black cutworm moth, Agrotis ipsilon. J Chem Ecol 5:439–457. https://doi.org/10.1007/BF00987929
Hoefele D, Chalissery JM, Gries R, Gries G (2020) Effects of trail pheromone purity, dose, and type of placement on recruiting European fire ants, Myrmica rubra, to food baits. J Entomol Soc Br Columbia 117:31–41
Hölldobler B, Wilson EO (1977) Colony-specific territorial pheromone in the African weaver ant Oecophylla longinoda (Latreille). Proc Natl Acad Sci 74:2072–2075. https://doi.org/10.1073/pnas.74.5.2072
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press
I’Anson Price R, Dulex N, Vial N et al (2019) Honeybees forage more successfully without the “dance language” in challenging environments. Sci adv 5:eaat0450
Ito Y, Hojo MK (2022) Cognitive control in trail-following ant foragers. IUSSI San Diego
Jeanne RL (1981) Alarm recruitment, attack behavior, and the role of the alarm pheromone in Polybia occidentalis (Hymenoptera: Vespidae). Behav Ecol Sociobiol 9:143–148
Kamin LJ (1968) “Attention-like” processes in classical conditioning. Miami
Lenth RV, Bolker B, Buerkner P et al (2023) emmeans: estimated marginal means, aka least-squares means
Linn M, Glaser SM, Peng T, Grüter C (2020) Octopamine and dopamine mediate waggle dance following and information use in honeybees. Proc R Soc B Biol Sci 287:20201950. https://doi.org/10.1098/rspb.2020.1950
Mackintosh NJ (1971) An analysis of overshadowing and blocking. Q J Exp Psychol 23:118–125. https://doi.org/10.1080/00335557143000121
Mackintosh NJ (1976) Overshadowing and stimulus intensity. Anim Learn Behav 4:186–192. https://doi.org/10.3758/BF03214033
Menzel R (2012) The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci 13:758–768. https://doi.org/10.1038/nrn3357
Menzel R, Giurfa M (2006) Dimensions of cognition in an insect, the honeybee. Behav Cogn Neurosci Rev 5:24–40. https://doi.org/10.1177/1534582306289522
Menzel R, Brembs B, Giurfa M (2006) Cognition in invertebrates. 403–422
Murmu MS, Hanoune J, Choi A et al (2020) Modulatory effects of pheromone on olfactory learning and memory in moths. J Insect Physiol 127:104159. https://doi.org/10.1016/j.jinsphys.2020.104159
Oberhauser FB, Koch A, Czaczkes TJ (2018) Small differences in learning speed for different food qualities can drive efficient collective foraging in ant colonies. Behav Ecol Sociobiol 72:164. https://doi.org/10.1007/s00265-018-2583-6
Oberhauser FB, Schlemm A, Wendt S, Czaczkes TJ (2019) Private information conflict: Lasius niger ants prefer olfactory cues to route memory. Anim Cogn 22:355–364. https://doi.org/10.1007/s10071-019-01248-3
Oberhauser FB, Bogenberger K, Czaczkes TJ (2022) Ants prefer the option they are trained to first. J Exp Biol 225:jeb243984. https://doi.org/10.1242/jeb.243984
Oberhauser FB, Wendt S, Czaczkes TJ (2020) Trail pheromone does not modulate subjective reward evaluation in Lasius niger ants. Front Psychol 11:
Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford Univ. Press, Oxford, England
Piqueret B, Sandoz J-C, d’Ettorre P (2019) Ants learn fast and do not forget: associative olfactory learning, memory and extinction in Formica fusca. R Soc Open Sci 6:190778. https://doi.org/10.1098/rsos.190778
Rossi N, d’Ettorre P, Giurfa M (2018) Pheromones modulate responsiveness to a noxious stimulus in honey bees. J Exp Biol 221:jeb172270
Rossi N, Pereyra M, Moauro MA et al (2020) Trail pheromone modulates subjective reward evaluation in Argentine ants. J Exp Biol 223:jeb230532. https://doi.org/10.1242/jeb.230532
Schaal B, Coureaud G, Langlois D et al (2003) Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424:68–72. https://doi.org/10.1038/nature01739
Schubert M, Sandoz J-C, Galizia G et al (2015) Odourant domininance in olfactory mixture processing: what makes a strong odourant? Proc R Soc B Biol Sci 282:20142562. https://doi.org/10.1098/rspb.2014.2562
Schürch R, Grüter C (2014) Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS One 9:e104660. https://doi.org/10.1371/journal.pone.0104660
Seenivasagan T, Vijayaraghavan R (2010) Chapter twenty-four - oviposition pheromones in haematophagous insects. In: Litwack G (ed) Vitamins & Hormones. Academic Press, pp 597–630
Seid MA, Traniello JFA (2005) Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Naturwissenschaften 92:198–201. https://doi.org/10.1007/s00114-005-0610-8
Shackleton K, Balfour NJ, Al Toufailia H et al (2023) Honey bee waggle dances facilitate shorter foraging distances and increased foraging aggregation. Anim Behav 198:11–19. https://doi.org/10.1016/j.anbehav.2023.01.009
Stowers L, Liberles SD (2016) State-dependent responses to sex pheromones in mouse. Curr Opin Neurobiol 38:74–79. https://doi.org/10.1016/j.conb.2016.04.001
Tait C, Naug D (2022) Interindividual variation in the use of social information during learning in honeybees. Proc R Soc B Biol Sci 289:20212501. https://doi.org/10.1098/rspb.2021.2501
Thom C, Gilley DC, Hooper J, Esch HE (2007) The scent of the waggle dance. PLoS Biol 5:e228. https://doi.org/10.1371/journal.pbio.0050228
Urlacher E, Francés B, Giurfa M, Devaud J-M (2010) An alarm pheromone modulates appetitive olfactory learning in the honeybee (Apis Mellifera). Front Behav Neurosci 4:
Vergoz V, Schreurs HA, Mercer AR (2007) Queen pheromone blocks aversive learning in young worker bees. Science 317:384–386. https://doi.org/10.1126/science.1142448
von Thienen W, Metzler D, Choe D-H, Witte V (2014) Pheromone communication in ants: a detailed analysis of concentration-dependent decisions in three species. Behav Ecol Sociobiol 68:1611–1627. https://doi.org/10.1007/s00265-014-1770-3
Wagner T, Galante H, Josens R, Czaczkes TJ (2022) A systematic examination of learning in the invasive ant Linepithema humile reveals very rapid development of short and long-term memory. 2022.04.12.487867
Wang Z, Qu Y, Dong S et al (2016) Honey bees modulate their olfactory learning in the presence of hornet predators and alarm component. PLoS One 11:e0150399. https://doi.org/10.1371/journal.pone.0150399
Wenig K, Bach R, Czaczkes TJ (2021) Hard limits to cognitive flexibility: ants can learn to ignore but not avoid pheromone trails. J Exp Biol 224:jeb242454. https://doi.org/10.1242/jeb.242454
Wenner AM, Wells PH, Johnson DL (1969) Honey bee recruitment to food sources: olfaction or language? Science 164:84–86. https://doi.org/10.1126/science.164.3875.84
Wickham H, Chang W, Henry L et al (2023) ggplot2: create elegant data visualisations using the grammar of graphics
Wissink M, Nehring V (2021) Appetitive olfactory learning suffers in ants when octopamine or dopamine receptors are blocked. J Exp Biol 224:jeb242732. https://doi.org/10.1242/jeb.242732
Wyatt TD (2017) Pheromones. Curr Biol 27:R739–R743. https://doi.org/10.1016/j.cub.2017.06.039
Funding
Open Access funding enabled and organized by Projekt DEAL. H2020 European Research Council,948181,Tomer J. Czaczkes,Deutsche Forschungsgemeinschaft,CZ 237 / 4-1,Tomer J. Czaczkes
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by M. Lihoreau
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection “Toward a Cognitive Ecology of Invertebrates” - Guest Editors: Aurore Avarguès-Weber and Mathieu Lihoreau
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koch, A., Kabas, M. & Czaczkes, T.J. No evidence that recruitment pheromone modulates olfactory, visual, or spatial learning in the ant Lasius niger. Behav Ecol Sociobiol 78, 16 (2024). https://doi.org/10.1007/s00265-024-03430-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03430-1