Abstract
Artificial light at night (ALAN) is a growing global problem altering the behavior of organisms and thereby community composition and ecosystem processes. Research has mostly focused on terrestrial ecosystems, but a growing number of studies show that aquatic ecosystems are increasingly affected. Here, we provide a conceptual framework that describes how ALAN can influence aquatic ecosystems through effects on the behavior of ecologically important invertebrates. These organisms provide a range of critical ecological functions, from serving as food for other organisms to nutrient cycling and the translocation of energy and matter within and between ecosystems. In addition, we systematically searched the literature to assess the current state of the field and identify knowledge gaps. The literature search reveals that an increasing number of studies find light pollution to alter the behavior of aquatic invertebrates, such as their movements, habitat choice, and foraging behavior, but that the fitness consequences of these behavioral changes are largely unknown, as are their impacts on populations, communities, and ecosystems. Yet, assessing the consequences of behavioral changes for higher ecological levels is of vital importance given the central role of these invertebrates in ecosystems. Thus, more research needs to be directed to the ecological consequences of behavioral responses of aquatic invertebrates to light pollution. Overall, more effort should be made to assess the ecological consequences of behavioral responses to ALAN, and, importantly, how negative effects of light pollution could be mitigated.
Significance statement
Light pollution is of growing ecological concern and influencing ecosystems through effects on the behavior of organisms. Aquatic ecosystems are increasingly exposed and an ecologically important group of organisms in these systems are invertebrates. Here, we discuss how artificial light at night alters the behavior of aquatic invertebrates and how this in turn influences ecosystem structure and function. Such an understanding of the mechanisms and pathways that underlie the effect of light pollution on aquatic ecosystems is needed if we are to develop efficient strategies to reduce negative effects of human-made lighting systems on ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increased use of artificial light at night (ALAN) is of growing concern given its impact on biodiversity and the functioning of ecosystems (Gaston et al. 2013; Holker et al. 2010; Longcore and Rich 2004). The topic has received increased attention since the key publications by Longcore and Rich in 2004 (Longcore and Rich 2004) and 2006 (Rice and Longcore 2006), but has mostly focused on effects in terrestrial ecosystems (Sanders et al. 2020). However, a few early papers documented negative effects on aquatic organisms (Moore et al. 2001; Verheijen 1958) and recent studies show that aquatic ecosystems are increasingly exposed to artificial light (Davies et al. 2020; Holker et al. 2021). In particular, organisms in waters close to human settlement, transport networks, and industry are exposed, such as in rivers, ponds and coastal waters, but also organisms in deeper waters and offshore areas, through skyglow and light from vessels and offshore infrastructures such as oil rigs (Davies et al. 2014). The intensity of illumination in these areas varies spatially and temporally, with lower levels on seafloor than in shallow urban ponds (Davies et al. 2020), and depending on daily and seasonal light cycles (Gaston et al. 2017; Massetti 2020). The effect of this variation on organisms depends in turn on the environmental conditions that the species have adapted to over evolutionary time (Tuomainen and Candolin 2011).
Ecologically important organisms in these light-exposed habitats are aquatic invertebrates; they nourish higher trophic levels, decompose organic matter, recycle nutrients, and translocate energy and matter within and between habitats and ecosystems. They are often sensitive to artificial light because many of their behaviors are set by light cycles, such as daily activities and seasonal migrations (Gaston et al. 2017). Thus, artificial light at night has the potential to influence their behaviors and, hence, their ecological functions.
To assess the impact that light pollution has on aquatic invertebrates and their ecological functions, an understanding of the underlying mechanisms and pathways is needed, as well as of the influencing factors, such as life histories and interactions with other human-inflicted disturbances. Such knowledge is needed if we are to develop efficient management strategies to reduce negative effects of light pollution on aquatic ecosystems.
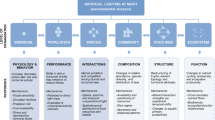
To facilitate research into this important field, we provide here a conceptual framework that illustrates the pathways and mechanisms through which light pollution influences the behavior of aquatic invertebrates and thereby their ecological functions (Fig. 1). We also assess the current state of the field by systematically searching the literature. We begin with giving an overview of the mechanisms and pathways through which artificial light influences the behavior of aquatic invertebrates. We then systematically search the literature to assess the current state of the field and to identify knowledge gaps. We continue with describing common behavioral responses of aquatic invertebrates to light pollution, illustrating with examples from the literature as well as expected effects based on ecological theory. We then move onto explaining the impact that behavioral responses can have on individual fitness, population dynamics, and community composition, and eventually on ecological conditions such as nutrient cycling and the translocation of energy and matter between disparate habitats. We end with discussing the research that is needed to advance this important field of research, and the knowledge that we need to acquire to develop efficient mitigation strategies.
A conceptual framework of the impact that artificial light can have on aquatic ecosystems through effects on the behavior of invertebrates. Changes in the behavior of individuals, such as habitat choice or foraging, can influence their population dynamics and interactions with other species, which, in turn, can influence the composition of the species community and thereby ecosystem structure and processes. Skyglow differs from direct light in that the level of irradiance is generally much lower
Mechanisms
Plastic responses and genetic adaptation
The first response of an animal to a change in the environment—such as light pollution—is often behavioral: the individual may alter its activity, habitat choice, food consumption etc. (Wong and Candolin 2015). These plastic responses are ultimately genetically determined and shaped by past environmental conditions and selection pressures (Sih et al. 2011; Tuomainen and Candolin 2011), but may be modified by experiences during lifetime and by transgenerational effects, i.e., when the experiences of parents, and possibly grandparents, influence offspring development and gene expression (Bell and Hellmann 2019). Given this dependence of responses on past conditions, behavioral responses to artificial light at night are often maladaptive, as species have not experienced similar conditions in their evolutionary past and, hence, are unlikely to have evolved adaptive reaction norms for responding to artificial nocturnal light (Robertson et al. 2013; Sih et al. 2011; Tuomainen and Candolin 2011). An example is the attraction of aquatic insects to streetlights when emerging from water bodies, which can drastically reduce their probability of survival (Manfrin et al. 2017, 2018).
When behavioral responses reduce the fitness of individuals and are maladaptive, new reaction norms are needed for the population to remain viable. Their evolution depends on a range of factors, such as the generation time of the species, the presence of genetic variation in the direction of selection, the rate at which new mutations arise, gene flow, and the size of the population (Bell 2017). Thus, species with longer generation time may not adapt fast enough to prevent population decline (Chevin and Lande 2010). Species with shorter generation time may be more likely, depending on the presence of genetic variation in the direction of selection, but little is currently known about the ability of aquatic invertebrates to adapt to light pollution.
Direct and indirect effects
Animals may respond to artificial light directly, or indirectly by responding to the reactions of other species or to the changes that the light causes to the abiotic environment (Wootton 1994). The responses can be immediate, such as when an individual changes its activity level or habitat use, or develop gradually over the lifetime of an individual, such as when physiological or morphological changes are needed, or a specific developmental stage needs to be reached before the behavior can be expressed (Buchanan and Partecke 2012; Wingfield 2013).
Indirect effects can in some instances be more profound than the direct effects, as species are linked to each other and to the environment through a myriad of interactions (Tylianakis et al. 2008). Thus, artificial light that alters the behavior of one species influences also the other species that it is linked with, and such effects can reach far beyond the directly affected species. Feedbacks among species and with the abiotic environment can further alter the ultimate effect of artificial light on species and result in complex changes to the community.
Influencing factors
The impact that artificial light has on aquatic invertebrates depends on a range of factors, such as the characteristics of the light and its temporal and spatial distribution, the transmission of the light within the water, the contrast to the background, water depth, and the structure of the habitat above and within the water body, as discussed in several recent publications (e.g., Davies et al. 2014; Tidau et al. 2021) (Table 1). In particular, the ongoing switch from narrow spectrum lamps to broad-spectrum LED lamps is expected to amplify negative effects of artificial light on organisms, as the LED light resembles natural day light more strongly than light from older lamps (Longcore et al. 2018).
Both direct illumination and sky glow from urban areas influence light conditions (Kyba et al. 2017). The impact can be further affected by habitat choice and the activity time of species; species in surface waters and more open habitats, and those emerging from hiding when night falls, are often more sensitive to light at night than burrowing species or species shielded by vegetation (Lynn et al. 2021). The responses can also depend on the circadian rhythm of the species, as diurnal species often extend their activity period and reduce the time for rest, while nocturnal species may show the opposite pattern (Sanders et al. 2020).
How a species responds to artificial light at night depends also on its visual system. This can vary from simple perception of the presence of light to the possibility of perceiving colors (Gaston et al. 2013). Aquatic species in dark habitats, such as in deeper waters, are often highly sensitive to artificial light (Gal et al. 1999). Some species are also able to perceive wavelengths that we humans cannot, as well as light polarization, which needs to be considered when assessing the effects of light pollution on animals (Marshall 2017; Marshall and Cronin 2011).
Review of the literature
To review the literature on the impact of artificial light on aquatic invertebrates and their ecological functions, we used the guidelines set out in the Collaboration for Environmental Evidence (CEE) and in RepOrting standards for Systematic Evidence Synthesis (ROSES) (Berger-Tal et al. 2019; Haddaway et al. 2018). We searched Web of Science using ‘All databases’ option with searches starting from 1945, using the search string ((“artificial night light*” OR “night-time light*” OR “nocturnal illumination” OR “outdoor light*” OR “artificial light” OR “light* at night*” OR “anthropogen* light*” OR “urban light*” OR “light pollution*” OR “nightlight*” OR “man made light*” OR “street light*” OR streetlight* OR “street lamp*” OR streetlamp* OR “sky glow*” OR “skyglow*”) AND (aquatic OR marine OR limnetic OR sea OR pelagic OR river* OR stream* OR lake* OR pond* OR water* OR coast* OR shore* OR intertidal OR reef* OR beach*) AND (invertebrate* OR insect* or amphipod* OR isopod* OR zooplankton OR snail* or mollusc* OR worm* OR shrimp* OR benth* OR anemone* OR crab* OR coral*)). This resulted in 304 publications, until 21 October 2022, with the earliest publications from 1973.
We screened the publications for inclusion criteria: (1) test for effects of artificial light at night on the behavior of one or more aquatic invertebrates either in the field or in the laboratory (excluding constant day and night light); (2) have a control in the form of before/after study or paired sites/treatments or use different light intensities or spectrum. This resulted in 76 publications that we categorized according to (1) the habitat of the species: benthos, plankton, nekton, intertidal, and species with terrestrial stages, (2) the behavior recorded: antipredator (including boldness), attraction to light, emergence (from water or from hosts), foraging, movements (including migration and habitat choice), and social behavior (including competition and reproduction), (3) whether the behavioral response was adaptive, maladaptive, or if adaptedness was unknown, and (4) the ecological level at which effects where recorded: individual, population, community, or ecosystem. The literature search is not exhaustive, but to keep the search objective and repeatable, we included only studies detected with the search string.
Inspecting the results, we find research effort to vary among taxa: insects with terrestrial stages and benthos have received most attention, while less attention has been paid to zooplankton (Fig. 2). Thus, the scope of the research should be broadened to more often consider species in the open water, as these are increasingly exposed to light pollution and important components of food webs (Davies et al. 2014). Attention also differs among behaviors; most research has focused on movements and attraction to artificial light, while antipredator and social behaviors, including reproductive behaviors, have gained less attention. Yet, these behaviors are critical determinants of fitness and influence population dynamics. Most worryingly, few studies have assessed the adaptive value of the recorded behavioral responses, i.e., whether the responses influence fitness components such as survival, growth, and reproduction. As a consequence, none of the included studies were able to determine the impact of the recorded behavioral responses on population dynamics. A few studies have documented effects of light pollution on community composition, but the mechanisms behind the effects are unknown, i.e., whether the changes in composition are related to changes in dispersal, mortality, or reproductive success of individual species. Information on indirect effects of artificial light, through changes in species interactions, are scarce, although these effects can be larger than the direct effects (Tylianakis et al. 2008). Finally, the consequences of behavioral responses for ecosystem processes are notable poorly known, such as decomposition, nutrient cycling, and the transmission of energy and matter within and between habitats and ecosystems. When conclusions are drawn, these are usually circumstantial. In addition, the duration of performed investigations is often short, such as short-term exposures of individuals to light in the lab or in the field, while longer term and gradually developing changes in behavior and their consequences for individual and higher ecological levels are unexplored. Thus, while our knowledge of the effects of light pollution on the behavior of aquatic invertebrates has been steadily growing during the last decades, worrying little is known about the consequences of these behavioral responses for populations, communities, and ecosystems, and especially so in the longer term.
Behaviors sensitive to artificial light
In the next sections, we discuss how artificial light at night is influencing, and expected to influence, various behaviors of aquatic invertebrates, drawing on examples from the literature and general ecological theory (Table 2). Behaviors sensitive to artificial light at night are expected to differ between day and night active species: sensitive behaviors in day active species are likely to be those that depend on vision, such as foraging, while sensitive behaviors in night active and crepuscular species are more likely to be behaviors that increase visibility to predators, such as general activity.
Biological timings
Many biological activities are set by internal clocks that are synchronized by natural light cycles, such as circadian, circalunar, and circannual clocks (Gaston et al. 2017). Circadian cycles are easily disrupted by artificial light, such as the nightly migration by zooplankton to surface waters (Cohen and Forward 2009), and the emergence of animals from their daily hiding places when night falls (Duarte et al. 2019; Torres et al. 2020).
Behaviors that are set by lunar cycles can similarly be altered by artificial light. An example is the synchronization of mass-spawning by corals that is set by moonlight and, hence, can be disrupted by artificial light (Lin et al. 2021). Artificial light can also alter seasonal activities—such as yearly migrations and the timing of reproduction—by extending the day and giving the impression of spring arriving earlier or autumn later. Such changes can in turn cause mismatches with other environmental conditions, such as with temperature or activities of other organisms. For instance, changes in the appearance of zooplankton in spring because of artificial light can cause mismatches with phytoplankton blooms, which in turn can alter the population dynamics of the zooplankton and, hence, the species that depend on the zooplankton as food (Asch et al. 2019).
Habitat choice and movements
Night-time illumination can alter the habitat choice of aquatic invertebrates through attraction (positive phototaxis) or repulsion (negative phototaxis) (Longcore and Rich 2004). Many nocturnal aquatic invertebrates are positively phototactic and artificial light can consequently cause an evolutionary trap, i.e., that they are attracted to the light because past selection has tuned the species to respond to light in such a way (Robertson et al. 2013). Light can also promote the emergence of aquatic insects from water bodies and influence their behavior after emergence. For instance, streetlights close to the water can double the number of insects emerging and draw them to artificially illuminated patches rather than to the surrounding habitat (Manfrin et al. 2017).
Negatively phototactic invertebrates avoid in turn illuminated areas. For instance, the sandy beach amphipod Americorchestia longicornis, which hides in the sediments in the day and emerges during the night to feed, is less inclined to emerge when exposed to artificial light, and when it emerges, it restricts its tidal distribution range if exposed to the light (Lynn et al. 2021). Species that use visual cues in their habitat choice can also have the choices altered by artificial light (Matsumura and Qian 2014). For instance, the larvae of several coral species select the depth for settlement depending on how the incoming light influences the appearance of the habitat (Mundy and Babcock 1998).
Artificial light has frequently been found to influence migration and dispersal. For instance, streetlights along streams reduce the drift of aquatic insects by increasing the time the insects spend hiding in the leaf litter or in the interstitial spaces between cobbles (Henn et al. 2014; Perkin et al. 2014). Zooplankton alter in turn their vertical position when exposed to night-time light (Berge et al. 2020). An example is light from bypassing ships that alters the distribution of arctic zooplankton during polar nights (Ludvigsen et al. 2018). Artificial light can also form barriers to dispersal and disrupt habitat connectivity, such as the illumination of only a part of a shore (Bennie et al. 2014), or disrupt navigation, as found for the sandhopper Talitrus saltator that uses moonlight as a compass during tidal migrations up and down the shore (Ugolini et al. 2005).
Foraging
Both foraging activity and foraging success can be sensitive to artificial light. Night active species may reduce their foraging activity, while day active species may extend foraging into the night, or initiate foraging earlier in the morning. Light can also improve the ability of individuals to orient in a habitat and increase the foraging success of both diurnal and crepuscular species (Longcore and Rich 2004).
Changes in foraging behavior can, in turn, influence the amount of food consumed or the nutritional value. For example, the snail Lymnea stagnalis increases its feeding rate under artificial light (Mondy et al. 2021). The light can also enhance foraging activity of herbivores by improving the abundance or palatability of primary producers, as found in terrestrial environments (Bennie et al. 2015). On the other hand, light sensitive species may reduce their foraging activity, as found for the night active sandy beach amphipod Orchestoidea tuberculate (Luarte et al. 2016) and the inshore mollusc Concholepas concholepas (Manriquez et al. 2021). Cannibalistic interactions can also be influenced by artificial light. An example is the intertidal burrowing crab Neohelice granulate where adults increase their cannibalism of juveniles when exposed to artificial light, probably because the juveniles are less able to escape the cannibalistic adults in lit environments (Nunez et al. 2021a).
Night-time light that induces stress and enhances energy demands increases again the need to feed (Swaddle et al. 2015). For instance, the dogwhelk Nucella lapillus increases its foraging activity while reducing its refuge-seeking behavior when exposed to night-time light (Underwood et al. 2017).
Predator avoidance
Artificial light at night can increases predation risk for night active species by eliminating the presence of darkness as a refuge. For instance, gammarids suffer higher predation pressure from fish in lit areas (Czarnecka et al. 2019). Similarly, insects emerging from water bodies experience higher predation pressure from terrestrial predators (Eisenbeis 2006). An increased risk of predation can in turn reduce the willingness of individuals to engage in fitness related activities, such as foraging and reproduction. For example, two crayfishes, the New River crayfish Cambarus chasmodactylus and the spiny stream crayfish Orconectes cristavarius, spend more time in shelter when exposed to night-time light (Fischer et al. 2020).
For day active species, artificial light at night can reduce predation risk by improving the ability to detect predators, or reducing the activity of their predators. On the other hand, the shortening, or even disappearance of the night, can cause stress and decrease physical condition or vigilance and, hence, make individuals easier prey. Little is, however, known about such effects for aquatic invertebrates.
The ability of species to melt into the background—camouflage – can be affected by artificial light and influence predation risk. A potential example is cephalopods that avoid predators by adjusting their body patterns to the immediate environment, the efficiency of which could be influenced by artificial light (Mathger et al. 2009). Bioluminescence signals that are used as defenses against predators can in turn be masked by artificial light and increase predation risk (Haddock et al. 2010). Ostracods, for instance, reduce bioluminescent signaling under artificial light, which could reduce their predation risk (Gerrish et al. 2009).
Reproduction
Artificial light can influence reproductive behaviors through effects on hormones, physical condition, general activity, and the possibility to find and evaluate potential mates (Buchanan and Partecke 2012; Candolin and Wong 2019; Elgert et al. 2020). Illumination often needs to be below a critical threshold before night active individuals engage in reproductive activities, while day active invertebrates may extend their reproductive activities into the night (Davies et al. 2014). Artificial light that decreases visibility and the background contrast of mate choice signals, such as bioluminescent signals, can in turn reduce the ability to locate and assess mates (Candolin 2019a; Gerrish et al. 2009; Rosenthal and Stuart-Fox 2012).
Artificial light that hampers the perception of natural light cycles may in turn disrupt the synchronization of reproductive activities. For instance, artificial light disturbs the moon-light dependent synchronization of gamete release in two coral species, Acropora millepora and A. digitifera (Ayalon et al. 2021b). Artificial light can also alter hormonal levels and thereby influence seasonal reproductive activities (Buchanan and Partecke 2012). For instance, increased perceived length of the day stimulates the production of an egg laying hormone in the pond snail Lymnaea stagnalis (Kitai et al. 2021).
The localization of oviposition sites by aquatic insects with terrestrial adult stages are again distorted by artificial light, as the insects use horizontally polarized light reflected off water bodies for finding suitable sites (Horvath et al. 2009; Schwind 1991). Instead, the insects may oviposit on human constructed surfaces that reflect horizontally polarized light, such as asphalt, bridges, windows, and cars (Egri et al. 2019; Kriska et al. 2008; Szaz et al. 2015).
Competition
Both intra- and interspecific competition can be affected by artificial light at night. Increased visibility can intensify agonistic interactions among individuals, or induce stress that reduces competitive ability (Navara and Nelson 2007). For instance, two crayfishes, Faxonious rusticus and F. virile, spend more time fighting when exposed to night-time light (Jackson and Moore 2019). Increases in general activity can in turn deplete energy reserves and intensify competition for food, while decreased activity may reduce encounters both within and between species and, hence, agonistic interactions. Changes in the efficiency of visual signals reflecting competitive ability can further influence competitive interactions, as documented for fishes (Candolin et al. 2014, 2016; Rosenthal and Stuart-Fox 2012), and similar effects may occur in aquatic invertebrates.
Competition among species is especially likely to be influenced by artificial light, as only one of the species needs to be affected. In addition, the impact of artificial light can be mediated by effects on a third species, such as altered abundance of the prey that two species are competing for (Hoover and Tylianakis 2012). Such alterations may be common but their effects on aquatic invertebrates still need to be documented.
Host-parasite interactions
Host-parasite interactions (including host–pathogen interactions) are ubiquitous and changes in the behavior of one of the species can influence the other species (Budria and Candolin 2014). Artificial light is especially likely to alter their encounter rate through effects on visibility, movements, and population sizes. For example, the encounter rate of trematodes with their ultimate hosts depends on light conditions that determine the release of the infective stages from their intermediate hosts, molluscs, and also their subsequent survival and infectivity (Pietrock and Marcogliese 2003). Similarly, changes in light conditions that alter daily or seasonal activity patterns of either hosts or parasites can alter their encounter rate and thereby infection rate. Yet, such effects remain to investigated for aquatic invertebrates.
The impact of artificial light on infectivity and resistance to infections can be mediated by impact on physiological processes, such as induce stress that reduces immunocompetence, or alter the abundance and quality of food and thereby influence physical condition (Lopez and Duffy 2021). Changes in infectivity and resistance can, in turn, alter the abundance of the interacting partners and their encounter rate, which can cause further changes to the host-parasite interaction. Such potential effects would deserve more attention given the profound effect that infections can have on population viability.
Mutualism and commensalism
Mutualistic and commensalistic interactions are essential components of species interaction webs, which contribute to maintain biological biodiversity and ecosystem stability (Stachowicz 2001). These interactions are likely to be sensitive to light pollution because of their high degree of specialization; species may not be able to switch to another partner if their main partner is negatively affected by light pollution (Hoover and Tylianakis 2012). An example is the symbiosis between corals and endosymbiotic algae that is sensitive to artificial light through negative effects on the photosynthetic performances of the algae (Ayalon et al. 2021a). Similarly, the mutualistic relationship of corals with fishes is likely to be sensitive to artificial light, as the light influences the survival and growth of coral reef fishes, as demonstrated for the orange-fin anemonefish Amphiprion chrysopterus (Schligler et al. 2021).
Mutualistic and commensalistic interactions can also be indirectly affected by artificial light, similarly to other interactions. For example, light pollution that attracts larger predatory fishes can increase predation pressure on smaller species (Becker et al. 2013), which in turn can alter their mutualistic and commensalistic relationships with other organisms. However, little is currently known about the impact of artificial light on mutualistic and commensalistic interactions of aquatic invertebrates, despite their expected ubiquity and important ecological function.
Impact of behavioral responses on individual fitness and population growth
Behavioral responses to artificial light that influence the fitness of individuals—their survival and reproductive success—can influence population dynamics (Fig. 1). The review of the literature reveals that research has so far focused on documenting behavioral responses of aquatic invertebrates to artificial light, while their fitness consequences have remained largely unknown (Fig. 2). Yet, behavioral responses can have profound fitness consequences, as suggested by a few studies. For instance, reduced foraging activity by night active amphipods and crabs reduces their growth rate, which could negatively impact their survival and reproduction (Czarnecka et al. 2021; Luarte et al. 2016; Nunez et al. 2021a, b). Increased activity under light pollution can in turn increase visibility to predators, as well as cause the allocation of energy away from growth and reproduction.
Another common behavioral response that is likely to influence fitness is the attraction of aquatic insects to artificial light. The aggregation of insects with terrestrial life stages around light sources increases their visibility to predators and reduces their foraging activity while increasing energy expenditure (Bolton et al. 2017; Eisenbeis 2006; Owens and Lewis 2018). Moreover, artificial light that hampers their detection of suitable oviposition sites when returning to water to reproduce can reduce reproductive success (Horvath et al. 2009; Kriska et al. 2008). Artificial light that obscures lunar cycles can again disrupt the synchronizing of mass spawnings in species that depend on the lights for the timing of the event, such as corals (Ayalon et al. 2021b).
Changes in individual fitness can, in turn, influence the size, structure and distribution of the population (Gaston and Bennie 2014; Pelletier and Garant 2012). Yet, we currently lack knowledge of such population consequences. Most evidence is circumstantial, such as the recent decline of the giant water bug Lethocerus deyrolli, which could be related to its attraction to artificial light, such as streetlight, which increases predation risk (Yoon et al. 2010).
Impact of behavioral responses on community composition
Changes in the behavior of one species influence other species via the network of species interactions (Hoover and Tylianakis 2012; Sanders and Gaston 2018). The impacts can be trait- or density-mediated, with changes in behavior, or in density because of behavioral responses, influencing directly and indirectly linked species (Fig. 1) (Werner and Peacor 2003).
A change in species interaction can initiate a cascade of effects that ripple through the ecological network of species interactions (Tylianakis et al. 2008). The cascade of effects can influence species that are not directly affected by the artificial light, while feedback responses and time lags can cause complex shifts to the species community (Bartley et al. 2019). Despite the obvious importance that species interactions play in determining community composition, the consequences of behavioral responses of aquatic invertebrates have received little attention, as revealed in our literature search (Fig. 2). Only a few studies have investigated the impact of light pollution on the composition of aquatic communities, and the contribution of altered behavior and species interactions are unexplored (Bolton et al. 2017; Davies et al. 2015; Garratt et al. 2019; Martin et al. 2021; Meyer and Sullivan 2013). Hence, the pathways and mechanisms behind community changes in light polluted areas are largely unknown.
Ecological ramifications
Behavioral responses to light pollution could influence ecosystem processes, such as nutrient cycling, bioturbation, carbon sequestering, primary production, and the flow of energy to higher trophic levels (Fig. 1) (Candolin 2019b; Palkovacs and Dalton 2012; Wilson et al. 2020; Zapata et al. 2019). Even habitats not directly exposed to the light could be affected through the movements of species. For instance, changes in the diel vertical migration of zooplankton is likely to alter the transport of energy and matter between surface and deeper water, as the zooplankton consume near-surface phytoplankton during the night and defecate fecal pellets in deeper water during the day (Hays 2003). Changes in their migration could also influence water clarity through altered predation on phytoplankton, as well as the transfer of energy and matter to higher trophic levels (Hays 2003).
Changes in diel movements of benthic species could similarly influence the transfer of energy and matter between habitats and also to higher trophic levels. An example is the change in diel movements of crabs and shrimps between seagrass beds and adjacent habitats, which may alter not only the transfer of energy and matter between these habitat but also the movements of their predators (Martin et al. 2021). Similarly, changes in the drift of invertebrates, and the choice of settlement habitat, can influence the flow of organic matter within water bodies, as well as the food availability to drift-feeding fishes, including game fishes such as salmonids (Baxter et al. 2005).
Changes in grazing or detritus feeding can in turn influence the recycling of energy and matter and thereby the biomass of primary producers and the length of food chains. An example is the change in detritus and litter feeding by freshwater amphipods exposed to artificial light, as this can alter the decomposition of organic matter and thereby the cycling of carbon and nutrients (Czarnecka et al. 2021).
Regarding the connection between aquatic and terrestrial ecosystems, increased emergence of aquatic insects from illuminated water bodies is likely to influence the flux of energy and matter between the systems (Manfrin et al. 2018; Perkin et al. 2011). Increased emergence could also influence the distribution and abundance of their terrestrial predators, such as bats, birds, and lizards, such as the concentration of aquatic insects around terrestrial artificial light sources (Baxter et al. 2005; Manfrin et al. 2017; Parkinson et al. 2020; Sullivan et al. 2019). Similarly, the aggregation of invertebrates in lit areas within aquatic ecosystems could influence the abundance and distribution of their predators. For instance, the recent shift towards smaller insects in light polluted habitats could be a consequence of the aggregation of fishes that prey on larger insects in these illuminated patches (Meyer and Sullivan 2013). Thus, while an increasing number of studies suggest that behavioral responses to artificial light could influence ecological processes, surprisingly little direct evidence exists (Fig. 2).
Interactions between light pollution and other disturbances
How behavioral responses to artificial light interact with other human-caused disturbances is generally poorly known (Swaddle et al. 2015). This is especially the case for aquatic ecosystems where the impact of light pollution has only recently received more interest. Interactions between disturbances can be additive or interactive (synergistic or antagonistic) and increase or lessen the effect of each stressor (Cote et al. 2016). Considering the number of anthropogenic disturbances that are currently impacting aquatic ecosystems, such interactions are likely to be common and could have profound effects on individuals and their ecological functions.
Studies on terrestrial ecosystems show that light pollution often interacts with noise pollution in influencing the behavior of individuals (Swaddle et al. 2015). Similar interactive effects could occur in aquatic ecosystems, as these are increasingly exposed to human-made noise. Climate change is another factor likely to interact with light pollution, particularly through effects on metabolism, stress levels, and the habitat choice of animals. An example is the tropical copepod Pseudodiaptomus incisus that reduces offspring production when light pollution is combined with rising water temperature, probably because the light increases stress levels (Nguyen et al. 2020). Interactions with rising temperature could also promote primary production and thereby influence the behavior of invertebrates, such as the foraging activity of grazers. The effect could be further amplified by anthropogenic eutrophication that also promotes primary production. Thus, many anthropogenic disturbances could be interacting in influencing the behavior and ecological function of invertebrates.
The invasion of foreign species is a growing ecological problem whose impact on ecosystems could be further enhanced by light pollution, particularly through negative effects on native species. Artificial light that reduces the abundance or competitive ability of native species could favor the invading species, and the effect could be further escalated by habitat degradation that alters the habitat choice, activity level, or physical condition of native species (Komine et al. 2020; Perkin et al. 2011; Thomas et al. 2016).
Finally, light is an important zeitgeber of many activities and interaction with disturbances in other zeitgebers, such as in temperature or water flow, could amplify the negative effects on organisms (Perkin and Wilson 2021). Such interactive effects could have profound implications for ecosystems given the importance of the correct timing of many activities for the fitness of individuals and the viability of populations.
Knowledge gaps and recommendations for future research
The review of the literature reveals that research has so far focused on examining the effects of artificial light on the behavior of aquatic invertebrates, while the consequences of these behavioral responses for populations, communities, and ecosystems are poorly known (Fig. 2). Yet, aquatic invertebrates are critical components of aquatic ecosystems that provide essential services, such as nutrient cycling, supporting higher trophic levels, and translocating energy and matter between habitats and ecosystems. Thus, changes in their behavior could have far-reaching consequences for ecosystem structure, function, and stability. Research is particularly needed on species that are known to play, or suspected to play, key roles in maintaining ecosystem function.
Below, we highlight a few areas especially in need of more research if we are to improve our knowledge of the impact that light pollution has on aquatic invertebrates and their ecological function, as well as the management actions needed to reduce negative effects on ecosystems.
Spatial effects of light pollution
Effects of artificial light could vary across areas and habitats depending on natural light cycles, intensities, and spectra. Regarding latitudes, more stable daily light cycles at lower latitudes could make species more sensitive to changes in these cycles, while the lower light intensities at higher latitudes could cause species to be sensitive to even low levels of artificial light. Similarly, water depth and light penetration are likely to influence the effects of light pollution: species in deeper water or more turbid water could be more sensitive to artificial light, given the naturally lower light intensities. Furthermore, spatial variation in light conditions within habitats could form barriers to movements and gene flow and influence migrating and dispersing species, while more sessile species may be unable to escape the light.
Temporal effects of light pollution
The time of the day or season when species are exposed to artificial light, and the duration of the exposure, are likely to influence responses. Crepuscular species are likely to be sensitive to light at twilight, while nocturnal species may be able to switch their activity times to later in the night (if light levels are lower then). The effect of artificial light may also depend on when during the life cycle they are exposed, and during which activities. For instance, exposure early in life could have more severe consequences than exposure later in life, while exposure during migration or when initiating reproductive activities could be more detrimental than during other times of life, given the profound impact these behaviors have on fitness.
Potential for genetic adaptation to light pollution
Information is scarce on the ability of species to adapt to light pollution. A terrestrial moth Yponomeuta cagnagella shows indications of adaptating to light pollution in terms of reduced flight-to-light behavior (Altermatt and Ebert 2016), but the degree to which aquatic invertebrates have been able to adapt to light pollution is unknown. Species are expected to differ in their potential depending on life history, population size, gene flow, and past light conditions and the evolutionary history of the population. The quality of the artificial light could further influence the potential to adapt, as light conditions that more strongly resemble day light—such as light from modern broadspectrum LEDs—could be more difficult to adapt to than the more yellow light from older lamps.
Influence of light pollution on species interactions
Species interactions determine ecosystem structure and function and more attention needs to be paid to the impact of light pollution on these interactions. Species with obligate interactions with only one or a few species are likely to be most sensitive to light pollution, as they cannot switch to other species if their main interaction partners are disturbed by the light. Species with higher flexibility could be more resilient, such as many invasive species.
Interactions among disturbances
More research is needed on how light pollution interacts with other anthropogenic disturbances, such as climate change, chemical pollution, noise, harvesting and fishing, and the invasion of new species. These other disturbances and stressor can amplify or curtail the effects of light pollution; failure to consider these interactions may hide the ultimate impact of light pollution on organisms and ecosystems.
Mitigation strategies
To limit the negative effects of light pollution on ecosystems, a range of solutions have been proposed, such as dimming or shielding light sources, limiting the illumination to desired areas and times, using motion-sensing lights that turn on and off as needed, and avoiding the use of light sources that resemble natural daylight, such as broadspectrum LEDs. The effectiveness and applicability of these measures have mainly concerned terrestrial ecosystems. Thus, efficient mitigation strategies need to be developed and evaluated also for aquatic ecosystems. In this endeavor, more information is particularly needed on the impact of the quality of artificial light and the time exposed.
Conclusions
The global spread of artificial light is eroding the natural night-time environment, which influences the behavior of both night and day active species. Given that behavior underlies interactions both within and between species, and with the abiotic environment, and these interactions determine biodiversity and ecological processes, assessing the impact of light pollution on behavior is of utmost importance. By systematically assessing the literature, we have shown that much evidence exists that light pollution influences various behaviors of aquatic invertebrates, from foraging to reproduction and the ability to avoid predators. Yet, little information exists on the fitness effects of these behavioral changes, or their consequences for populations, communities, and ecosystem and, hence, for biodiversity and the functioning of aquatic ecosystems. Thus, we call for more research into the ecological consequences of behavioral responses of aquatic invertebrates to artificial light at night. Aquatic invertebrates are key components of ecosystems and changes in their ecological functions could have far-reaching consequences for ecosystem structure, function, and stability. To facilitate the research, we have provided a conceptual framework that indicates the pathways and mechanisms through which light pollution can influence the behavior of aquatic invertebrates and thereby higher ecological levels. In addition, we have outlined the major gaps in our knowledge and the research that is needed to advance the field. Light pollution is fairly easily reversed and it leaves behind no residual effect, but the recovery may still take time depending on connectivity between habitats and colonization rates. Yet, negative effects of artificial light can be mitigated if we put our minds to it.
Data availability
All data generated and analyzed during this study are included in the supplementary information file.
References
Altermatt F, Ebert D (2016) Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol Lett 12:4. https://doi.org/10.1098/rsbl.2016.0111
Asch RG, Stock CA, Sarmiento JL (2019) Climate change impacts on mismatches between phytoplankton blooms and fish spawning phenology. Glob Change Biol 25:2544–2559. https://doi.org/10.1111/gcb.14650
Ayalon I, Benichou JIC, Avisar D, Levy O (2021a) The endosymbiotic coral algae symbiodiniaceae are sensitive to a sensory pollutant: Artificial Light at Night, ALAN. Front Physiol 12. https://doi.org/10.3389/fphys.2021.695083
Ayalon I et al (2021b) Coral gametogenesis collapse under artificial light pollution Curr Biol 31:413-+. https://doi.org/10.1016/j.cub.2020.10.039
Bartley TJ et al (2019) Food web rewiring in a changing world. Nat Ecol Evol 3:345–354. https://doi.org/10.1038/s41559-018-0772-3
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220. https://doi.org/10.1111/j.1365-2427.2004.01328.x
Becker A, Whitfield AK, Cowley PD, Jarnegren J, Naesje TF (2013) Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J Appl Ecol 50:43–50. https://doi.org/10.1111/1365-2664.12024
Bell AM, Hellmann JK (2019) An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. In: Futuyma DJ (ed) Annual Review of Ecology Evolution and Systematics, vol 50, pp 97–118. https://doi.org/10.1146/annurev-ecolsys-110218-024613
Bell G (2017) Evolutionary Rescue. Ann Rev Ecol Evol Syst 48:605–627. https://doi.org/10.1146/annurev-ecolsys-110316-023011
Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ (2015) Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil Trans R Soc B 370. https://doi.org/10.1098/rstb.2014.0131
Bennie J, Davies TW, Inger R, Gaston KJ (2014) Mapping artificial lightscapes for ecological studies. Methods Ecol Evol 5:534–540. https://doi.org/10.1111/2041-210x.12182
Berge J et al (2020) Artificial light during the polar night disrupts Arctic fish and zooplankton behaviour down to 200 m depth. Commun Biol 3. https://doi.org/10.1038/s42003-020-0807-6
Berger-Tal O et al (2019) Systematic reviews and maps as tools for applying behavioral ecology to management and policy. Behav Ecol 30:1–8. https://doi.org/10.1093/beheco/ary130
Bessell-Browne P, Negri AP, Fisher R, Clode PL, Duckworth A, Jones R (2017) Impacts of turbidity on corals: The relative importance of light limitation and suspended sediments. Mar Pollut Bull 117:161–170. https://doi.org/10.1016/j.marpolbul.2017.01.050
Bolton D, Mayer-Pinto M, Clark GF, Dafforn KA, Brassil WA, Becker A, Johnston EL (2017) Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci Total Environ 576:1–9. https://doi.org/10.1016/j.scitotenv.2016.10.037
Buchanan KL, Partecke J (2012) The endocrine system: can homeostasis be maintained in a changing world? In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world. Mechanisms and consequences. Oxford University Press, Oxford, pp 32-45
Budria A, Candolin U (2014) How does human-induced environmental change influence host-parasite interactions? Parasitology 141:462–474. https://doi.org/10.1017/s0031182013001881
Candolin U (2019a) Mate choice in a changing world. Biol Rev 94:1246–1260. https://doi.org/10.1111/brv.12501
Candolin U (2019) The threespine stickleback (Gasterosteus aculeatus) as a modifier of ecological disturbances. Evol Ecol Res 20:167–191
Candolin U, Nieminen A, Nyman J (2014) Indirect effects of human-induced environmental change on offspring production mediated by behavioural responses. Oecologia 174:87–97. https://doi.org/10.1007/s00442-013-2752-2
Candolin U, Tukiainen I, Bertell E (2016) Environmental change disrupts communication and sexual selection in a stickleback population. Ecology 97:969–979. https://doi.org/10.1890/15-1090.1
Candolin U, Wong BBM (2019) Mate choice in a polluted world: consequences for individuals, populations and communities. Phil Trans R Soc B 374:20180055. https://doi.org/10.1098/rstb.2018.0055
Chevin LM, Lande R (2010) When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64:1143–1150. https://doi.org/10.1111/j.1558-5646.2009.00875.x
Cohen JH, Forward RB (2009) Zooplankton diel veritical migration - a review of proximate control. In: Gibson RN, Atkinson RJA, Gordon JDM (eds) Oceanography and Marine Biology: An Annual Review, vol 47. Crc Press-Taylor & Francis Group, Boca Raton, pp 77–109
Cote IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc Lond B 283:9.https://doi.org/10.1098/rspb.2015.2592
Czarnecka M, Kakareko T, Jermacz L, Pawlak R, Kobak J (2019) Combined effects of nocturnal exposure to artificial light and habitat complexity on fish foraging. Sci Total Environ 684:14–22. https://doi.org/10.1016/j.scitotenv.2019.05.280
Czarnecka M, Kobak J, Grubisic M, Kakareko T (2021) Disruptive effect of artificial light at night on leaf litter consumption, growth and activity of freshwater shredders. Sci Total Environ 786. https://doi.org/10.1016/j.scitotenv.2021.147407
Davies TW, Coleman M, Griffith KM, Jenkins SR (2015) Night-time lighting alters the composition of marine epifaunal communities. Biol Lett 11. https://doi.org/10.1098/rsbl.2015.0080
Davies TW, Duffy JP, Bennie J, Gaston KJ (2014) The nature, extent, and ecological implications of marine light pollution. Front Ecol Environ 12:347–355. https://doi.org/10.1890/130281
Davies TW, McKee D, Fishwick J, Tidau S, Smyth T (2020) Biologically important artificial light at night on the seafloor. Sci Rep 10. https://doi.org/10.1038/s41598-020-69461-6
Diamantopoulou C, Christoforou E, Dominoni DM, Kaiserli E, Czyzewski J, Mirzai N, Spatharis S (2021) Wavelength-dependent effects of artificial light at night on phytoplankton growth and community structure. Proc R Soc Lond B 288:10. https://doi.org/10.1098/rspb.2021.0525
Duarte C et al (2019) Artificial light pollution at night (ALAN) disrupts the distribution and circadian rhythm of a sandy beach isopod. Environ Pollut 248:565–573. https://doi.org/10.1016/j.envpol.2019.02.037
Egri A, Szaz D, Pereszlenyi A, Bernath B, Kriska G (2019) Quantifying the polarised light pollution of an asphalt road: an ecological trap for the stonefly, Perla abdominalis (Guerin-Meneville, 1838) (Plecoptera: Perlidae). Aquat Insects 40:257–269. https://doi.org/10.1080/01650424.2019.1601228
Eisenbeis G (2006) Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In: Rice C, Longcore T (eds) Ecological Consequences of Artificial Night Lighting. Island Press, Washington D.C., pp 281–304
Elgert C, Hopkins J, Kaitala A, Candolin U (2020) Reproduction under light pollution: maladaptive response to spatial variation in artificial light in a glow-worm. Proc R Soc Lond B 287:7. https://doi.org/10.1098/rspb.2020.0806
Fischer JR, Gangloff MM, Creed RP (2020) The behavioral responses of 2 Appalachian crayfish to cool and warm spectrum LED lights at night Freshwater. Science 39:39–46. https://doi.org/10.1086/707459
Gal G, Loew ER, Rudstam LG, Mohammadian AM (1999) Light and diel vertical migration: spectral sensitivity and light avoidance by Mysis relicta. Can J Fish Aquat Sci 56:311–322. https://doi.org/10.1139/cjfas-56-2-311
Garratt MJ, Jenkins SR, Davies TW (2019) Mapping the consequences of artificial light at night for intertidal ecosystems. Sci Total Environ 691:760–768. https://doi.org/10.1016/j.scitotenv.2019.07.156
Gaston KJ, Bennie J (2014) Demographic effects of artificial nighttime lighting on animal populations. Environ Rev 22:323–330. https://doi.org/10.1139/er-2014-0005
Gaston KJ, Bennie J, Davies TW, Hopkins J (2013) The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol Rev 88:912–927. https://doi.org/10.1111/brv.12036
Gaston KJ, Davies TW, Nedelec SL, Holt LA (2017) Impacts of artificial light at night on biological timings Annual Review of Ecology. Evolution, and Systematics 48:49–68. https://doi.org/10.1146/annurev-ecolsys-110316-022745
Gerrish GA, Morin JG, Rivers TJ, Patrawala Z (2009) Darkness as an ecological resource: the role of light in partitioning the nocturnal niche. Oecologia 160:525–536. https://doi.org/10.1007/s00442-009-1327-8
Gjerlov C, Richardson J (2010) Experimental increases and reductions of light to streams: effects on periphyton and macroinvertebrate assemblages in a coniferous forest landscape. Hydrobiologia 652:195–206. https://doi.org/10.1007/s10750-010-0331-7
Haddaway NR, Macura B, Whaley P, Pullin AS (2018) ROSES RepOrting standards for Systematic Evidence Syntheses: pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ Evid 7. https://doi.org/10.1186/s13750-018-0121-7
Haddock SHD, Moline MA, Case JF (2010) Bioluminescence in the Sea. Ann Rev Mar Sci 2:443–493. https://doi.org/10.1146/annurev-marine-120308-081028
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170. https://doi.org/10.1023/B:HYDR.0000008476.23617.b0
Henn M, Nichols H, Zhang YX, Bonner TH (2014) Effect of artificial light on the drift of aquatic insects in urban central Texas streams. J Freshw Ecol 29:307–318. https://doi.org/10.1080/02705060.2014.900654
Holker F, Kuhne JL, Jechow A, van Grunsven RHA (2021) Impact of different colors of artificial light at night on phototaxis in aquatic insects. Integr Comp Biol 61:E385–E386
Holker F, Wolter C, Perkin EK, Tockner K (2010) Light pollution as a biodiversity threat. Trends Ecol Evol 25:681–682. https://doi.org/10.1016/j.tree.2010.09.007
Hoover SER, Tylianakis JM (2012) Species interactions. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford, pp 129–142
Horvath G, Kriska G, Malik P, Robertson B (2009) Polarized light pollution: a new kind of ecological photopollution. Front Ecol Environ 7:317-325.https://doi.org/10.1890/080129
Jackson KM, Moore PA (2019) The intensity and spectrum of artificial light at night alters crayfish interactions. Mar Freshw Behav Physiol 52:131–150. https://doi.org/10.1080/10236244.2019.1663124
Jechow A, Kollath Z, Ribas SJ, Spoelstra H, Holker F, Kyba CCM (2017) Imaging and mapping the impact of clouds on skyglow with all-sky photometry. Sci Rep 7. https://doi.org/10.1038/s41598-017-06998-z
Kitai H, Kakuda U, Goto SG, Shiga S (2021) Photoperiod controls egg laying and caudodorsal cell hormone expression but not gonadal development in the pond snail Lymnaea stagnalis. J Comp Physiol A 207:523–532. https://doi.org/10.1007/s00359-021-01494-2
Komine H, Koike S, Schwarzkopf L (2020) Impacts of artificial light on food intake in invasive toads. Sci Rep 10. https://doi.org/10.1038/s41598-020-63503-9
Kriska G, Bernath B, Farkas R, Horvath G (2009) Degrees of polarization of reflected light eliciting polarotaxis in dragonflies (Odonata), mayflies (Ephemeroptera) and tabanid flies (Tabanidae). J Insect Physiol 55:1167–1173. https://doi.org/10.1016/j.jinsphys.2009.08.013
Kriska G, Malik P, Szivak I, Horvath G (2008) Glass buildings on river banks as “polarized light traps” for mass-swarming polarotactic caddis flies. Naturwissenschaften 95:461–467. https://doi.org/10.1007/s00114-008-0345-4
Kyba CCM et al (2017) Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv 3:8. https://doi.org/10.1126/sciadv.1701528
Kyba CCM, Ruhtz T, Fischer J, Holker F (2011) Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. Plos One 6. https://doi.org/10.1371/journal.pone.0017307
Lin CH, Takahashi S, Mulla AJ, Nozawa Y (2021) Moonrise timing is key for synchronized spawning in coral Dipsastraea speciosa. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2101985118
Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2:191–198. https://doi.org/10.2307/3868314
Longcore T, Rodriguez A, Witherington B, Penniman JF, Herf L, Herf M (2018) Rapid assessment of lamp spectrum to quantify ecological effects of light at night. J Exp Zool Part A-Ecol Integr Physiol 329:511–521. https://doi.org/10.1002/jez.2184
Lopez LK, Duffy MA (2021) Mechanisms by which predators mediate host-parasite interactions in aquatic systems. Trends Parasitol 37:890–906. https://doi.org/10.1016/j.pt.2021.06.006
Luarte T, Bonta CC, Silva-Rodriguez EA, Quijon PA, Miranda C, Farias AA, Duarte C (2016) Light pollution reduces activity, food consumption and growth rates in a sandy beach invertebrate. Environ Pollut 218:1147–1153. https://doi.org/10.1016/j.envpol.2016.08.068
Ludvigsen M et al (2018) Use of an Autonomous Surface Vehicle reveals small-scale diel vertical migrations of zooplankton and susceptibility to light pollution under low solar irradiance. Sci Adv 4:8. https://doi.org/10.1126/sciadv.aap9887
Lynn KD et al (2021) Artificial light at night alters the activity and feeding behaviour of sandy beach amphipods and pose a threat to their ecological role in Atlantic Canada. Sci Total Environ 780. https://doi.org/10.1016/j.scitotenv.2021.146568
Manfrin A et al (2017) Artificial light at night affects organism flux across ecosystem boundaries and drives community structure in the recipient ecosystem Frontiers in Environmental. Science 5:61. https://doi.org/10.3389/fenvs.2017.00061
Manfrin A et al (2018) Dietary changes in predators and scavengers in a nocturnally illuminated riparian ecosystem. Oikos 127:960–969. https://doi.org/10.1111/oik.04696
Manriquez PH et al (2021) Effects of artificial light at night and predator cues on foraging and predator avoidance in the keystone inshore mollusc Concholepas concholepas. Environ Pollut 280. https://doi.org/10.1016/j.envpol.2021.116895
Marshall J (2017) Vision and lack of vision in the ocean. Curr Biol 27:R494–R502. https://doi.org/10.1016/j.cub.2017.03.012
Marshall J, Cronin TW (2011) Polarisation vision. Curr Biol 21:R101–R105. https://doi.org/10.1016/j.cub.2010.12.012
Martin CW, Reynolds LK, Scheffel WA, Tiffany S, Kopetman S (2021) Diel variability and influence of artificial light on fish and macroinvertebrate communities in Gulf of Mexico seagrass beds. Estuaries Coasts 44:431–441. https://doi.org/10.1007/s12237-020-00865-3
Massetti L (2020) Drivers of artificial light at night variability in urban, rural and remote areas. J Quant Spectrosc Radiat Transf 255. https://doi.org/10.1016/j.jqsrt.2020.107250
Maszczyk P, Talanda J, Babkiewicz E, Leniowski K, Urban P (2021) Daphnia depth selection in gradients of light intensity from different artificial sources: An evolutionary trap? Limnol Oceanogr 66:1367–1380. https://doi.org/10.1002/lno.11691
Mathger LM, Denton EJ, Marshall NJ, Hanlon RT (2009) Mechanisms and behavioural functions of structural coloration in cephalopods. J R Soc Interface 6:S149–S163. https://doi.org/10.1098/rsif.2008.0366.focus
Matsumura K, Qian PY (2014) Larval vision contributes to gregarious settlement in barnacles: adult red fluorescence as a possible visual signal. J Exp Biol 217:743–750. https://doi.org/10.1242/jeb.096990
McMahon TA, Rohr JR, Bernal XE (2017) Light and noise pollution interact to disrupt interspecific interactions. Ecology 98:1290–1299. https://doi.org/10.1002/ecy.1770
Meyer LA, Sullivan SMP (2013) Bright lights, big city: influences of ecological light pollution on reciprocal stream-riparian invertebrate fluxes. Ecol Appl 23:1322–1330. https://doi.org/10.1890/12-2007.1
Mondy N et al (2021) Herbivory increases on freshwater plants exposed to artificial light at night. Aquat Bot 175. https://doi.org/10.1016/j.aquabot.2021.103447
Moore MV, Pierce SM, Walsh HM, Kvalvik SK, Lim JD (2001) Urban light pollution alters the diel vertical migration of Daphnia. In: 27th Congress of the International-Association-of-Theoretical-and-Applied-Limnology, Dublin, Ireland, 1998 2001. International Association of Theoretical and Applied Limnology - Proceedings. E Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, pp 779–782
Mundy CN, Babcock RC (1998) Role of light intensity and spectral quality in coral settlement: Implications for depth-dependent settlement? J Exp Mar Biol Ecol 223:235–255. https://doi.org/10.1016/s0022-0981(97)00167-6
Navara KJ, Nelson RJ (2007) The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43:215–224. https://doi.org/10.1111/j.1600-079X.2007.00473.x
Navarro-Barranco C, Hughes LE (2015) Effects of light pollution on the emergent fauna of shallow marine ecosystems: Amphipods as a case study. Mar Pollut Bull 94:235–240. https://doi.org/10.1016/j.marpolbul.2015.02.023
Nguyen TT, Le MH, Doan NX, Pham HQ, Vu MTT, Dinh KV (2020) Artificial light pollution increases the sensitivity of tropical zooplankton to extreme warming. Environ Technol Innov 20. https://doi.org/10.1016/j.eti.2020.101179
Nunez JD, Bas CC, Garcia MP, Ocampo EH, Ribeiro PD, Luppi TA (2021a) Artificial light at night may increase the predation pressure in a salt marsh keystone species. Mar Environ Res 167. https://doi.org/10.1016/j.marenvres.2021.105285
Nunez JD, Sbragaglia V, Spivak ED, Chiaradia NM, Luppi TA (2021b) The magnitude of behavioural responses to artificial light at night depends on the ecological context in a coastal marine ecosystem engineer. Mar Environ Res 165. https://doi.org/10.1016/j.marenvres.2020.105238
Owens ACS, Lewis SM (2018) The impact of artificial light at night on nocturnal insects: A review and synthesis. Ecol Evol 8:11337–11358. https://doi.org/10.1002/ece3.4557
Palkovacs EP, Dalton CM (2012) Ecosystem consequences of behavioural palsticity and contemporary evolution. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world. Mechansims and consequences. Oxford University Press, Oxford, pp 175-189
Parkinson E, Lawson J, Tiegs SD (2020) Artificial light at night at the terrestrial-aquatic interface: Effects on predators and fluxes of insect prey. Plos One 15. https://doi.org/10.1371/journal.pone.0240138
Pelletier F, Garant D (2012) Population consequences of individual variation in behaviour. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world. Mechansims and consequences. Oxford University Press, Oxford, pp 159-174
Perkin EK, Holker F, Richardson JS, Sadler JP, Wolter C, Tockner K (2011) The influence of artificial light on stream and riparian ecosystems: questions, challenges, and perspectives Ecosphere 2. https://doi.org/10.1890/es11-00241.1
Perkin EK, Holker F, Tockner K, Richardson JS (2014) Artificial light as a disturbance to light-naive streams. Freshw Biol 59:2235–2244. https://doi.org/10.1111/fwb.12426
Perkin EK, Wilson MJ (2021) Anthropogenic alteration of flow, temperature, and light as life-history cues in stream ecosystems. Integr Comp Biol 61:1134–1146. https://doi.org/10.1093/icb/icab024
Pietrock M, Marcogliese DJ (2003) Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol 19:293–299. https://doi.org/10.1016/s1471-4922(03)00117-x
Rice C, Longcore T (eds) (2006) Ecological consequences of artificial night lighting. Island Press, Washington
Robertson BA, Rehage JS, Sih A (2013) Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol 28:552–560. https://doi.org/10.1016/j.tree.2013.04.004
Rosenthal GG, Stuart-Fox D (2012) Environmental disturbance and animal communication. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world: mechanisms and consequences. Oxford Unviersity Press, Oxford, pp 16–31
Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ (2020) A meta-analysis of biological impacts of artificial light at night. Nat Ecol Evol 10. https://doi.org/10.1038/s41559-020-01322-x
Sanders D, Gaston KJ (2018) How ecological communities respond to artificial light at night. J Exp Zool Part A-Ecol Integr Physiol 329:394–400. https://doi.org/10.1002/jez.2157
Schligler J, Cortese D, Beldade R, Swearer SE, Mills SC (2021) Long-term exposure to artificial light at night in the wild decreases survival and growth of a coral reef fish. Proc R Soc B Biol Sci 288. https://doi.org/10.1098/rspb.2021.0454
Schwind R (1991) Polarization vision in water insects and insects living on a moist substrate. J Comp Physiol A-Sens Neural Behav Physiol 169:531–540
Secondi J, Davranche A, Thery M, Mondy N, Lengagne T (2020) Assessing the effects of artificial light at night on biodiversity across latitude - Current knowledge gaps. Glob Ecol Biogeogr 29:404–419. https://doi.org/10.1111/geb.13037
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387. https://doi.org/10.1111/j.1752-4571.2010.00166.x
Smyth TJ et al (2021) A global atlas of artificial light at night under the sea. Elem-Sci Anthropocene 9. https://doi.org/10.1525/elementa.2021.00049
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. Bioscience 51:235–246. https://doi.org/10.1641/0006-3568(2001)051[0235:Mfatso]2.0.Co;2
Sullivan SMP, Hossler K, Meyer LA (2019) Artificial lighting at night alters aquatic-riparian invertebrate food webs. Ecol Appl 29. https://doi.org/10.1002/eap.1821
Swaddle JP et al (2015) A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol Evol 30:550–560. https://doi.org/10.1016/j.tree.2015.06.009
Szaz D et al (2015) Lamp-Lit Bridges as Dual Light-Traps for the Night-Swarming Mayfly, Ephoron virgo: Interaction of Polarized and Unpolarized Light Pollution. Plos One 10. https://doi.org/10.1371/journal.pone.0121194
Thomas JR, James J, Newman RC, Riley WD, Griffiths SW, Cable J (2016) The impact of streetlights on an aquatic invasive species: Artificial light at night alters signal crayfish behaviour Appl Anim. Behav Sci 176:143–149. https://doi.org/10.1016/j.applanim.2015.11.020
Tidau S et al (2021) Marine artificial light at night: An empirical and technical guide. Methods Ecol Evol 12:1588–1601. https://doi.org/10.1111/2041-210x.13653
Torres D, Tidau S, Jenkins S, Davies T (2020) Artificial skyglow disrupts celestial migration at night. Curr Biol 30:E696–E697. https://doi.org/10.1016/j.cub.2020.05.002
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11:1351–1363. https://doi.org/10.1111/j.1461-0248.2008.01250.x
Ugolini A, Boddi V, Mercatelli L, Castellini C (2005) Moon orientation in adult and young sandhoppers under artificial light. Proc R Soc Lond B 272:2189–2194. https://doi.org/10.1098/rspb.2005.3199
Underwood CN, Davies TW, Queiros AM (2017) Artificial light at night alters trophic interactions of intertidal invertebrates. J Anim Ecol 86:781–789. https://doi.org/10.1111/1365-2656.12670
Verheijen FJ (1958) The mechanisms of the trapping effect of artificial light sources upon animals. Arch Néerl Zool 13:1–107
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. https://doi.org/10.1890/0012-9658(2003)084[1083:arotii]2.0.co;2
Wilson MW, Ridlon AD, Gaynor KM, Gaines SD, Stier AC, Halpern BS (2020) Ecological impacts of human-induced animal behaviour change. Ecol Lett 23:1522–1536. https://doi.org/10.1111/ele.13571
Wingfield JC (2013) The comparative biology of environmental stress: behavioural endocrinology and variation in ability to cope with novel, changing environments. Anim Behav 85:1127–1133. https://doi.org/10.1016/j.anbehav.2013.02.018
Wong BBM, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26:665–673. https://doi.org/10.1093/beheco/aru183
Wootton JT (1994) The nature and consequences of indirect effects in ecological communities. Ann Rev Ecol Syst 25:443–466. https://doi.org/10.1146/annurev.es.25.110194.002303
Yoon TJ, Kim DG, Kim SY, Jo SI, Bae YJ (2010) Light-attraction flight of the giant water bug, Lethocerus deyrolli (Hemiptera: Belostomatidae), an endangered wetland insect in East Asia. Aquat Insects 32:195–203. https://doi.org/10.1080/01650424.2010.508045
Yoshimura M, Kubota T (2022) Evaluation of sunlight penetration through riparian forest and its effects on stream biota. Glob Ecol Conserv 34. https://doi.org/10.1016/j.gecco.2022.e02043
Zapata MJ, Sullivan SMP, Gray SM (2019) Artificial lighting at night in estuaries: Implications from individuals to ecosystems. Estuar Coasts 42:309–330. https://doi.org/10.1007/s12237-018-0479-3
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. Financial support was provided by the Finnish National Agency for Education, EDUFI.
Finnish National Agency for Education
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Since the research involved no primary data collection from living human or non-human participants, ethical approval was not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. C Choe
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganguly, A., Candolin, U. Impact of light pollution on aquatic invertebrates: Behavioral responses and ecological consequences. Behav Ecol Sociobiol 77, 104 (2023). https://doi.org/10.1007/s00265-023-03381-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03381-z