Abstract
We evaluated the hypothesis that social play behavior influences the development of temperament in young animals, using docility as a measure of temperament. We observed the play behavior of juvenile Belding’s ground squirrels (Urocitellus beldingi) during the developmental period in which play primarily occurs and conducted behavioral tests measuring docility at the beginning and toward the end of the play interval. Tests involved handling squirrels and recording their responses. We observed a significant but weak association between body mass and docility at the beginning of the play period, suggesting that docility may vary with size or energetic variables. Docility decreased significantly among juveniles over the play interval, and rates of social play were reliable predictors of change in docility. Juveniles who played at higher rates tended to have greater decreases in docility over the play interval, suggesting that social play might refine temperament toward more active responses in U. beldingi. Rates of social play among juveniles were reliable predictors of their scores on docility tests as yearlings, suggesting that possible effects of juvenile play on docility may extend beyond the juvenile period. Among mothers of juveniles in the study, docility during gestation and lactation were reliable predictors of docility after emergence of young from the natal burrow. However, docility of mothers decreased significantly between gestation and emergence of young, suggesting that although squirrels have individual tendencies toward docility, the expression of these tendencies may be influenced by behavioral context.
Significance statement
This study helps to elucidate ways in which juvenile social play influences the development of young animals. Various studies have suggested that juvenile play influences motor, social, and cognitive development. Here, we show an association between juvenile social play and development of temperament in Belding’s ground squirrels (Urocitellus beldingi). Juveniles who engaged in play at higher rates across the play interval had greater behavioral shifts from passive toward active responses. Refinements in temperament toward more proactive behavior might have benefits for young animals such as better preparing them to explore, investigate, and gather information about their social and physical environments as they venture away from their natal areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Play occurs in a range of animal taxa but is especially prominent in mammals and serves as an important component of juvenile development in an array of mammalian species (Bekoff and Byers 1998; Burghardt 2005; Pellis et al. 2015). Pellis et al. (2023) observed in rats (Rattus norvegicus) that social elements of play behavior can importantly influence development of executive functions mediated through the prefrontal cortex in mammals, resulting in increased social competence and greater behavioral flexibility and adaptability during adulthood. Along this line, Spinka et al. (2001) suggested that juvenile social play in mammals may fine tune development of temperament in ways that promote emotional stability, behavioral flexibility, and expression of adaptive behavior across a range of situations and circumstances. Elements of temperament can exhibit plasticity in various species during the juvenile period when behavior is developing and can be shaped by social, physiological, and ecological factors (Sinn et al. 2008; Petelle et al. 2017; DiRienzo et al. 2018; Cabrera et al. 2021). In this work, we studied a population of Belding’s ground squirrels (Urocitellus beldingi) to evaluate the relationship between juvenile social play behavior and the development of temperament. We used docility as a measure of temperament to assess whether play behavior might shape or refine elements of temperament in young animals. Behaviors along the docility spectrum of temperament are generally considered to reflect reactive inclinations that involve gathering information before acting or proactive tendencies related to taking immediate action (Réale et al. 2000, 2009; Petelle et al. 2013). Appropriate expression of proactive and reactive responses may be important to juvenile U. beldingi as they gather information about their physical and social environments and also during important events later in life such as emigrating from the natal area or defending territories (Nunes and Monroy Montemayor 2023).
Temperament is also referred to as personality and includes behavioral tendencies of individuals that are consistent and repeatable over time and across situations (Sih et al. 2004; Réale et al. 2007). Temperaments occur along spectra that, for example, can encompass responses to risks or threats (shyness/caution–boldness) or responses to novel objects or situations (avoidance–exploration). Behaviors along the docility spectrum are generally measured as responses to being trapped and handled and reflect tendencies toward proactive vs. reactive behavior rather than responses specifically to threats or novelty (Réale et al. 2000, 2007, 2009; Cooper 2009; Herde and Eccard 2013; Petelle et al. 2013). For example, Petelle et al. (2015) observed that docility in yellow-bellied marmots (Marmota flaviventris) was inversely related to activity during open field tests but not related to boldness when individuals were confronted with a potential threat. Docile individuals tend to be inclined toward expressing less active behavior across situations or to wait and gather information before acting (Sih et al. 2004; Petelle et al. 2013, 2015, 2017).
Early-life experiences can have important and long-lasting effects on the development of temperament (Rӧdel and von Hulst 2009; Rӧdel and Monclús 2011; de Jong et al. 2022). Elements of temperament can be stable across an individual’s lifespan or can exhibit varying degrees of plasticity. For example, Petelle et al. (2013) observed in yellow-bellied marmots that tendencies toward boldness in response to a threat were consistent among individuals early in life, whereas tendencies toward docility tended to be more consistent across the lifespan. Moreover, temperament can affect a range of ecologically important variables such as physiological responses to stress, space use, dispersal, reproductive success, and survival and thus can have an impact on the ability of animals to navigate through the life challenges they face (Réale et al. 2007, 2010; Boon et al. 2008; Baugh et al. 2013; Clary et al. 2014; Wauters et al. 2021; Skinner et al. 2022). Temperament can also have important fitness consequences for individuals, and different temperaments can be adaptive under different environmental conditions, varying with factors such as the prevalence of predators, abundance of resources, or local population density (Dingemanse et al. 2004; Both et al. 2005; Boon et al. 2007; Colchester and Harrison 2016; Rasmussen and Belk 2017; Vetter et al. 2016). For example, in stable environments, proactive individuals who are less docile and respond quickly to environmental stimuli may have an advantage. By contrast, in unstable or unpredictable environments, reactive individuals who are more docile and gather information before acting may be favored by natural selection (Sih et al. 2004).
The expression of juvenile play behavior and the adaptive benefits of engaging in play can vary among species and between sexes within a species (Olioff and Stewart 1978; Meder 1990; Pederson et al. 1990; Pellis et al. 1996; Bekoff and Byers 1998; Nunes et al. 1999; Maestripieri and Ross 2004; Burghardt 2005; Paukner and Suomi 2008; Auger and Olesen 2009; Wang et al. 2021). Juvenile play can have benefits that are experienced prominently early in the life of individuals. For example, in horses (Equus caballus), juvenile play is associated with enhanced body condition and increased survival (Cameron et al. 2008). Play in white-tailed deer (Odocoileus virginianus) and mule deer (Odocoileus hemionus) promotes development of anti-predator behavior and increases behavioral versatility among juveniles (Carter et al. 2019). Play in geladas (Theropithecus gelada) improves motor skill and fosters development of social relationships (Gallo et al. 2021). In brown bears (Ursus arctos), play among cubs facilitates increased survival to the following summer, possibly by relieving stress and reducing stress-related sources of mortality (Fagen and Fagen 2004). In U. beldingi, play behavior promotes development of motor skill and may contribute to earlier natal dispersal in young males (Nunes et al. 2004). Juvenile play can also have a range of long-term benefits that extend beyond the juvenile period. For example, laboratory rats make extensive hand movements during play that likely promote long-term improvement of their motor and manipulation skills (Whishaw et al. 2021). In spotted hyenas (Crocuta crocuta), play can facilitate social assessment by providing young animals with information about clan members with whom they will interact throughout their lives (Nolfo et al. 2021). Play behavior in yellow-bellied marmots helps to establish dominance relationships that persist into adulthood (Blumstein et al. 2013). Rough and tumble play in juvenile American minks (Neovison vison) is correlated with longer copulations among males in adulthood and longer periods of precopulatory activity among females possibly facilitating mate choice (Ahloy Dallaire and Mason 2017). Juvenile play in female U. beldingi is associated with greater intensity of maternal territorial behavior as a yearling and increased likelihood of establishing a maternal territory and weaning a litter (Nunes 2014). In brown bears, play behavior as a juvenile is associated with increased long-term survival (Fagen and Fagen 2009).
Prior work with U. beldingi suggested a relationship between juvenile social play and development of temperament along the exploration–avoidance axis. Juvenile squirrels who engaged in social play at higher rates and had longer play interactions had greater increases across the developmental period in which play primarily occurs in their tendencies to explore an unfamiliar testing arena (Marks et al. 2017). Shifts in young animals toward greater exploratory behavior may in general promote greater acquisition of information as they venture farther from their natal areas or begin to integrate themselves into their social groups (Nunes and Monroy Montemayor 2023). Here, we expanded on the prior work of Marks et al. (2017) to evaluate whether juvenile social play in U. beldingi might be associated with development of other elements of temperament, focusing on temperament along the docility spectrum. We observed the play behavior of juvenile U. beldingi and evaluated other factors that might influence the development of temperament in young animals, such as body mass and the docility of their mothers (Mousseau and Fox 1998; Rӧdel and von Hulst 2009). We conducted behavioral tests assessing docility at the beginning and toward the end of the primary developmental play interval for U. beldingi. We predicted that if social play influences the development of docility, then social play in individuals should be correlated with their changes in docility across the play interval.
Methods
Study animals and general methods
From May to June 2016 and June to August 2017, we studied a population of U. beldingi in a 25-ha meadow at Tioga Pass (latitude 37.9, longitude −119.2, elevation 2950 m) in Mono County, CA, USA. Squirrels in this species live in groups but do not have a complex social system compared to many other sciurid species (Michener 1983; Wolff and Sherman 2007). The squirrels are diurnal, inhabit alpine and subalpine meadows in the western USA, and have an 8–9-month hibernation period interspersed with 3–4-month active periods that coincide with the annual growing season (Jenkins and Eshelman 1984). Mating begins shortly after emergence from hibernation, and females bear at most one litter per year (Morton and Gallup 1975). Males do not begin breeding until they are 2 years old, whereas females may begin reproducing as yearlings (Jenkins and Eshelman 1984). Reproductive females defend maternal territories and aggressively evict intruders from the territories (Nunes et al. 2000). Gestation lasts 24–25 days. During lactation, young remain underground in natal burrows and first emerge above ground at 25–28 days of age, near the time of weaning (Holekamp et al. 1984; Nunes et al. 1999). Play behavior occurs primarily during juveniles’ first 2 weeks above ground, with > 97% of play interactions occurring among littermates (Nunes et al. 1999).
We captured squirrels using Tomahawk live traps (Tomahawk Live Trap Company, Hazelhurst, WI, USA) baited with peanut butter and checked traps every 30 min or less during trapping sessions. Squirrels were fitted with numbered metal ear tags (National Band and Tag Company, Newport, KY, USA) at their first capture for long-term identification. Ear tags of juveniles were painted different colors with nail polish prior to application to aid in identification of individuals during observations (Nunes et al. 2015). We also dyed the fur of squirrels with unique symbols using black hair dye (Clairol, Stamford, CT, USA) to facilitate visual identification of individuals. We observed the territories of reproductive females daily to determine the dates that their litters first emerged from the natal burrow. We trapped young within 2 days of their first emergence above ground when they could be unambiguously assigned to mothers (Holekamp et al. 1984). We weighed juveniles with spring balance scales (Avinet, Dryden, NY, USA) at their first capture and again 12–14 days after their first appearance above ground and calculated changes in body mass across this period as a proportion of initial mass. Methods used in the study followed guidelines for wild mammals published by the American Society of Mammalogists (Sikes et al. 2016).
Behavioral tests
We conducted behavioral tests to evaluate docility of U. beldingi. During tests, a handler removed a squirrel from its trap, and the squirrel was held for 30 s with the handler’s hand positioned around the squirrel’s chest and abdomen and the squirrel’s forelimbs free from the handler’s grip (Fig. 1). Squirrels were allowed to equilibrate in a trap covered by a cloth for at least 2 min in a quiet area before tests began. Squirrels’ responses during tests were recorded on video, and recordings were later viewed by observers to determine docility scores. Scores were calculated as the number of seconds during the 30-s test that squirrels were docile. Docility was defined as remaining motionless or displaying slight movements such as turning the head or repositioning the paws (Fig. 1A). Squirrels were not considered to be docile if they wriggled their bodies or otherwise struggled, used their forelimbs to push down on the handler’s glove to try to lift their body free from the handler’s grip, or bit the handler’s glove (Fig. 1B). All recordings were scored blindly by the same two observers, and scores for individual squirrels were averaged.
We conducted docility tests for 151 juveniles from 32 litters at their initial capture after first emerging from the natal burrow. Docility tests were conducted before any other handling procedures such as ear-tagging, dye-marking, or weighing were performed to prevent the possibility of these procedures influencing responses on tests. We observed the behavior (see below) of 90 of these juveniles from 19 litters during their first 12 days above ground and conducted a second docility test for these 90 juveniles 12–14 days after their initial emergence from the natal burrow, toward the end of the period in which play primarily occurs. We note that ten juveniles from nine of the 19 litters disappeared before they could be re-tested and were omitted from this part of the study.
To evaluate whether possible effects of juvenile play on docility extended beyond the play period, we conducted docility tests on 23 yearling U. beldingi from 14 different litters for whom juvenile play data were available from the previous year. To evaluate whether docility was consistent among individual U. beldingi in different contexts, we conducted docility tests during different phases of the reproductive cycle for 30 adult and yearling females who weaned a litter during the study. Tests were performed at the female’s first capture in the active season either during gestation or lactation and then again within the first week after emergence of young from the natal burrow. Lactation was defined as the 27-day interval prior to the first appearance of young above ground, and gestation was defined as the 25-day interval prior to lactation (Holekamp et al. 1984; Nunes et al. 1999).
Observation of juvenile behavior
During the summer of 2016, we observed the play behavior of 23 juvenile squirrels from 14 different litters who were included in the study as yearlings in 2017. These squirrels were observed as juveniles for an average of 396 + 16.2 (SE) min per individual over an average of 5.9 ± 0.2 (SE) different days during their first 12 days above ground. During the summer of 2017, we observed the behavior of 90 juvenile U. beldingi from 19 litters. These juveniles were observed for an average of 484.7 + 19.0 (SE) min per individual over an average of 6.1 ±0.3 (SE) different days during their first 12 days above ground. Behavioral observations were conducted between 0700 and 1600 h from elevated posts such as boulders and hillsides. We recorded all occurrences of social play during observations and calculated rates of social play for individuals as the number of play interactions per hour of observation. Specific play behaviors were defined (Table 1) following descriptions by Marks et al. (2017). We observed specific focal animals, so it was not possible to record behavioral data blindly.
Data analysis
Continuous variables evaluated in the study included scores on docility tests, changes in docility scores, body mass, changes in body mass, and rates of juvenile social play. Correlations between variables were evaluated with Pearson’s r. Data were compared between juvenile males and females using independent t-tests. Scores on docility tests for individual U. beldingi at the beginning vs. end of the play interval were compared using paired t-tests. Variances were pooled when assumptions of homoscedasticity were not met in t-tests. A linear mixed effects model was used to evaluate whether docility of juveniles at first emergence from the natal burrow varied with their body mass or the docility of their mothers. A linear mixed effects model was also used to evaluate whether changes in docility of juveniles across the play interval varied with rates of social play, body mass, changes in body mass, or the docility of their mothers. Litter was included as a random effect in linear mixed effects models to account for possible similarities among littermates. Linear regression was used to evaluate whether docility scores of reproductive females prior to emergence of young from the natal burrow were reliable predictors of docility scores after litter emergence and whether rates of social play among juvenile squirrels were reliable predictors of their scores on docility tests as yearlings. Assumptions of homoscedasticity and normal distribution of residuals were met in statistical analyses. Statistical tests were performed with Systat 13.1 (Systat Software Inc., Chicago, IL, USA). Relationships indicated by statistical tests were considered significant when P < 0.05.
Results
Initial docility of juveniles
The amount of time juvenile U. beldingi were docile during behavioral tests at their first emergence from the natal burrow did not differ between the sexes (t149 = 0.53, P = 0.597), so data for juvenile males and females were combined for statistical analysis. Body mass of juveniles at first emergence from the natal burrow also did not differ between the sexes (t149 = 0.80, P = 0.428) and was not significantly correlated with docility of mothers (r = −0.01, P = 0.947). Juvenile docility during initial behavioral tests varied significantly with the body mass of juveniles but not with the docility of their mothers (Table 2). Docility of juveniles tended to decrease as their body mass increased, but the association between these variables was weak (Fig. 2).
Changes in juvenile docility
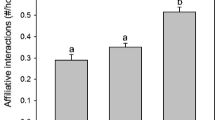
The amount of time juveniles were docile during behavioral tests decreased significantly between initial tests at first emergence from the natal burrow and re-tests near the end of the play period (Fig. 3, t89 = 10.55, P < 0.001), indicating a shift toward more active responses across the play interval. Changes in docility scores over the play interval, expressed as a proportion of the initial score, did not differ between juvenile males and females (t88 =1.18, P = 0.241). Scores on initial tests were significantly correlated with scores on re-tests (r = 0.53, P < 0.001) but not with changes in docility over the play interval (r = −0.13, P = 0.223).
Box and whisker plots showing docility scores of 151 individual juvenile U. beldingi near their first emergence from the natal burrow at the onset of the play interval and 12–14 days after emerging from the natal burrow near the end of the play interval. Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 interquartile range, and outliers are plotted as individual points. Letters indicate statistical differences at P < 0.05
Changes in docility of juveniles varied significantly with social play but not with average body mass across the play interval, changes in body mass across the play interval, or docility scores of mothers (Table 3). Higher rates of social play were associated with greater decreases in docility and greater shifts toward active responses (Fig. 4). We note that there were no significant sex differences in rates of play (t88 = 1.31, P = 0.194), body mass (t88 = 0.45, P = 0.653), or changes in body mass (t88 = 1.33, P = 0.188), and none of the independent variables in this analysis was significantly correlated with any others (−0.24 < r < 0.16, 0.124 < P < 0.999). We further note that although changes in docility varied with rates of social play, the rates of social play were not significantly correlated with initial docility scores (r = −0.15, P = 0.159).
Docility of yearlings and reproductive females
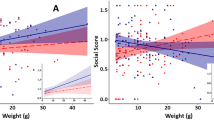
To evaluate possible longer-term associations between docility and juvenile play, docility tests were conducted on 23 yearling U. beldingi for whom juvenile play data from the previous year were available. Among these squirrels, the rates of social play as a juvenile were reliable predictors of scores on docility tests as a yearling (Fig. 5, F1,21 = 8.64, P = 0.008, R2 = 0.26). In particular, squirrels who engaged in social play at higher rates as juveniles tended to be less docile as yearlings (Fig. 5).
To evaluate consistency in docility among individual U. beldingi over time and across situations, we conducted docility tests for a total of 30 reproductive females both prior to and after the first emergence of their litters from the natal burrow. Among these females, 12 were first tested during gestation, and 18 were first tested during lactation. Docility scores increased significantly between the initial test and re-test for females first tested during gestation (Fig. 6, t11 = 3.17, P = 0.009) but not for those first tested during lactation (t17 = 0.75, P = 0.465). Scores on initial docility tests were reliable predictors of scores on the re-tests for females first tested during gestation (Fig. 7A, F1,10 = 13.32, P = 0.004, R2 = 0.53) as well as those first tested during lactation (Fig. 7B, F1,16 = 16.49, P = 0.001, R2 = 0.48).
Box and whisker plots showing docility scores of 12 female Urocitellus beldingi during the reproductive cycle. Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 interquartile range, and outliers are plotted as individual points. Letters indicate a significant difference between stages of the reproductive cycle at P < 0.05
Association between docility scores of reproductive female Urocitellus beldingi after first emergence of litters from the natal burrow and scores during A lactation and B gestation. Twelve females were sampled during gestation and after emergence of young, and 18 females were sampled during lactation and after emergence of young
Discussion
Body mass of juvenile U. beldingi in our study was inversely related to docility at juveniles’ first emergence from the natal burrow, with smaller juveniles having more docile and less active responses during behavioral tests. Body mass has been shown to influence the developmental trajectory of behavioral tendencies in European rabbits (Oryctolagus cuniculus), including exploratory and aggressive behavior and responses to restraint (Rӧdel and von Hulst 2009; Rӧdel and Monclús 2011; Rӧdel et al. 2017). Body mass in juvenile U. beldingi at first emergence from the natal burrow is associated with the size of fat reserves (Nunes et al. 1999). Smaller juveniles tend to have less body fat and thus might be inclined to be more conservative in their energy usage, and development of more docile temperaments might be favored in these individuals. Moreover, smaller juveniles might be less competitive in agonistic interactions and thus might be favored to have more docile and reactive responses rather than proactive behavior (Sih et al. 2004; Rӧdel and von Hulst 2009). We note, however, that the association between body mass and docility in juvenile U. beldingi was weak (Fig. 2), and possible influences of body mass on docility may be relatively minor. Another possibility is that body mass of U. beldingi at emergence from the natal burrow reflected the expression of temperament during lactation. That is, more docile juveniles might have been less likely to seek nursing opportunities or might have been less successful at competing for milk and thus might have been smaller near the time of weaning (Rӧdel et al. 2017).
We did not observe a significant relationship between the docility of maternal U. beldingi and the docility of their young. However, we cannot rule out here the possibility that other maternal effects influenced the development of docility in juvenile U. beldingi in our study. Temperament is plastic during the postnatal and juvenile periods in a range of species (Cabrera et al. 2021), and maternal effects on the development of temperament and other variables have been suggested to prepare offspring to be successful under prevailing environmental conditions (Storm and Lima 2010; Kapheim et al. 2011; Dantzer et al. 2013). Environmental stresses experienced by mothers can directly affect the way they raise their young, which in turn can affect the behavior as well as growth, metabolism, and immune and endocrine function of offspring (Mousseau and Fox 1998; Weinstock 2001; Hayward and Wingfield 2004). For example, environmental stressors and prior experience raising young have been observed to influence glucocorticoid concentrations in mothers, and glucocorticoids passed to offspring through milk during lactation can influence development of behavior and elements of temperament such as boldness and docility (Hinde et al. 2015; Petelle et al. 2017).
Docility among juvenile U. beldingi in our study decreased over the course of the play interval, with the behavior of juveniles shifting toward more active responses. Marks et al. (2017) observed that exploratory behavior of juvenile U. beldingi increased across the play interval, and the amount of time to escape from an unfamiliar testing arena decreased. Juvenile U. beldingi are unfamiliar with above-ground elements of their habitat when they first emerge from the natal burrow, and reactive responses such as passive observation might help naïve squirrels avoid potentially dangerous situations and thus might be favored by natural selection. However, as young squirrels spend time above ground and gain familiarity with their surroundings, exploration and proactive responses might be favored to help individuals gather additional information about and navigate through their physical and social environments as they begin to venture farther from their natal home areas (Sih et al. 2004).
We observed a significant relationship between social play and decreases in docility of juvenile U. beldingi after emergence from the natal burrow. Juveniles who played at higher rates tended to have greater decreases in docility and greater shifts toward active responses in behavioral tests. Marks et al. (2017) similarly observed that juvenile U. beldingi who engaged in social play at higher rates tended to have greater increases in exploratory behavior after emergence from the natal burrow. Shehan (2019) further observed that greater social play among juvenile U. beldingi was associated with greater increases in caution in response to a potential threat. Thus, temperament appears to be plastic in U. beldingi during the juvenile period. Moreover, possible refinements in different elements of temperament associated with social play in U. beldingi might better equip young squirrels to express adaptive responses across a diverse array of situations they may encounter as they interact with the world around them.
Social play of U. beldingi as juveniles in this study was significantly correlated with their docility as yearlings, suggesting that possible effects of social play on docility may extend beyond the juvenile period in this species. Play behavior has been shown to have a variety of adaptive benefits during the juvenile period as well as longer-term effects on behavior, reproductive success, and survival in a variety of species (Bekoff and Byers 1998; Burghardt 2005; Cameron et al. 2008; Fagen and Fagen 2009; Blumstein et al. 2013; Nunes 2014; Ahloy Dallaire and Mason 2017; Whishaw et al. 2021). However, we note that studies of meerkats (Suricata suricatta) (Sharpe and Cherry 2003; Sharpe 2005a, b, c) and chimpanzees (Pan troglodytes schweinfurthii) (Heintz et al. 2017) did not reveal any associations between juvenile play and behavior later in life. These discrepancies in longer-term effects of play may reflect different courses in the evolution of play behavior among different species (Pellis et al. 2014).
Among maternal U. beldingi in our study, docility scores of individual females during gestation and lactation were reliable predictors of their docility after young emerged from the natal burrow, indicating consistency among individuals in their tendencies toward docile responses and aligning docility in this species with Réale et al.’s (2007) definition of temperament. However, we observed a significant increase in the docility of maternal U. beldingi between gestation and the emergence of their young from the natal burrow, indicating that although individual females may be predisposed to different degrees of docility, expression of docility can be influenced by factors such as reproductive state and may vary across different contexts. Maternal U. beldingi exhibit peak aggressive behavior during the gestational period when they are competing for burrow systems and space in which to establish a maternal territory, and rates of aggression decrease substantially after gestation (Nunes et al. 1997, 2000). Thus, changes in docility across the reproductive cycle may reflect increases in aggressive behavior or general tendencies toward more proactive responses when establishing a maternal territory.
The possible effects of social play on the development temperament along the docility spectrum may be mediated by effects on the developing brain. Some elements of brain development are plastic during the postnatal and juvenile periods, and a range of experiences after birth can play an important role in directing specific aspects of neural and behavioral development (Johnson 2001; Stiles and Jernigan 2010; Kolb and Gibb 2011; Sakai and Sugiyama 2018). For example, in rats, social play experiences during early development are necessary for the establishment of competent social skills and the normal expression of social behavior in adulthood (Pellis et al. 2014; Stark and Pellis 2020; Stark et al. 2021). Juvenile play in rats has also been shown to modify development of areas in the frontal cortex associated with motor and social behavior as well as behavioral flexibility (Bell et al. 2010; Pellis et al. 2010; Himmler et al. 2013, 2017; Burleson et al. 2016).
Pellis et al. (2019) proposed that in some species, play may comprise a behavioral system devoted to the expression and regulation of play behaviors. This idea suggests that play arose evolutionarily as non-functional expression during early development of behaviors that are functional in adulthood. Over time, these behaviors were modified by natural selection into specific play behaviors that provided various adaptive benefits to individuals and that were regulated by distinct neural systems (Burghardt 2005; Pellis et al. 2014, 2015). Pellis et al. (2019) noted that in some species, behavior systems related to play may be complex. Social play can be diverse and can involve planning interactions with other individuals as well as responding to the actions of play partners and unfamiliar circumstances that arise during play interactions (Pellis and Pellis 2017; Palagi 2018). We suggest social play in juvenile U. beldingi may reinforce behavioral responses and that a function of play in this species might be to refine development of temperament and promote responses that help young animals maneuver through their surroundings and social situations they may encounter.
Data availability
The data reported in this paper are available in a supplementary information file.
References
Ahloy Dallaire J, Mason GM (2017) Rough-and-tumble play predicts adult sexual behavior in American mink. Anim Behav 123:81–89. https://doi.org/10.1016/j.anbehav.2016.10.023
Auger AP, Olesen KM (2009) Brain sex differences and the organization of juvenile social play behaviour. J Neuroendocrinol 21:519–525. https://doi.org/10.1111/j.1365-2826.2009.01871.x
Baugh AT, van Oers K, Naguib M, Hau M (2013) Initial reactivity and magnitude of the acute stress response associated with personality in wild great tits (Parus major). Gen Comp Endocrinol 189:96–104. https://doi.org/10.1016/j.ygcen.2013.04.030
Bekoff M, Byers JA (1998) Animal play: evolutionary, comparative, and ecological processes. Cambridge University Press, Cambridge, UK
Bell HC, Pellis SM, Kolb B (2010) Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res 2017:7–13. https://doi.org/10.1016/j.bbr.2009.09.029
Blumstein DT, Chung LK, Smith JE (2013) Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc R Soc B 280:20130485. https://doi.org/10.1098/rspb.2013.0485
Boon AK, Réale D, Boutin S (2007) The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol Lett 10:1094–1104. https://doi.org/10.1111/j.1461-0248.2007.01106.x
Boon AK, Réale D, Boutin S (2008) Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117:1321–1328. https://doi.org/10.1111/j.0030-1299.2008.16567.x
Both C, Dingemanse NJ, Drent PJ, Tinbergen JM (2005) Pairs of extreme avian personalities have highest reproductive success. J Anim Ecol 74:667–674. https://doi.org/10.1111/j.1365-2656.2005.00962.x
Burghardt GM (2005) The genesis of animal play: testing the limits. MIT Press, Cambridge, MA, USA
Burleson CA, Pedersen RW, Seddighi S, DeBusk LE, Burghardt GM, Cooper MA (2016) Social play in juvenile hamsters alters dendritic morphology in the medial prefrontal cortex and attenuates effects of social stress in adulthood. Behav Neurosci 130:437–447. https://doi.org/10.1037/bne0000148
Cabrera D, Nilsson JR, Griffen BD (2021) The development of animal personality across ontogeny: a cross-species review. Anim Behav 173:137–144. https://doi.org/10.1016/j.anbehav.2021.01.003
Cameron EZ, Linklater WL, Stafford KJ, Minot EO (2008) Maternal investment results in better foal condition through increased play behavior in horses. Anim Behav 76:1511–1518. https://doi.org/10.1016/j.anbehav.2008.07.009
Carter RN, Romanow CA, Pellis SM, Lingle S (2019) Play for prey: do deer fawns play to develop species-typical tactics or to prepare for the unexpected. Anim Behav 156:31–40. https://doi.org/10.1016/j.anbehav.2019.06.032
Clary D, Skyner LJ, Ryan CP, Gardiner LE, Anderson WG, Hare JF (2014) Shyness–boldness, but not exploration, predicts glucocorticoid stress response in Richardson’s ground squirrels (Urocitellus richardsonii). Ethology 120:1101–1109. https://doi.org/10.1111/eth.12283
Colchester C, Harrison NM (2016) Personality in blue tits (Cyanistes caeruleus) and its effect on their breeding success. Ethology 122:695–701. https://doi.org/10.1111/eth.12516
Cooper WE Jr (2009) Variation in escape behavior among individuals of the striped plateau lizard Sceloporus virgatus may reflect differences in boldness. J Herpetol 43:495–502. https://doi.org/10.1670/08-197R1.1
Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG (2013) Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340:1215–1217. https://doi.org/10.1126/science.1235765
de Jong M, Phillips BL, Llewelyn J, Chapple DG, Wong BBM (2022) Effects of developmental environment of animal personality in a tropical skink. Behav Ecol Sociobiol 76:137. https://doi.org/10.1007/s00265-022-03240-3
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc Lond B 271:847–852. https://doi.org/10.1098/rspb.2004.2680
DiRienzo N, Johnson JC, Dornhaus A (2018) Juvenile social experience generates differences in behavioral variation but not averages. Behav Ecol 30:455–464. https://doi.org/10.1093/beheco/ary185
Fagen R, Fagen J (2004) Juvenile survival and benefits of play behaviour in brown bears, Ursus arctos. Evol Ecol Res 6:89–102
Fagen R, Fagen J (2009) Play behaviour and multi-year juvenile survival in free-ranging brown bears, Ursus arctos. Evol Ecol Res 11:1053–1067
Gallo A, Caselli M, Norscia I, Palagi E (2021) Let’s unite in play! Play modality and group membership in wild geladas. Behav Process 184:104338. https://doi.org/10.1016/j.beproc.2021.104338
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to yolk and may alter offspring growth and phenotype. Gen Comp Endocrinol 135:365–371. https://doi.org/10.1016/j.ygcen.2003.11.002
Heintz MR, Murray CM, Markham AC, Pusey AE, Lonsdorf EV (2017) The relationship between social play and developmental milestones in wild chimpanzees (Pan troglodytes schweinfurthii). Am J Primatol 79:e22716. https://doi.org/10.1002/ajp.22716
Herde A, Eccard JA (2013) Consistency in boldness, activity and exploration at different stages of life. BMC Ecol 13:49. https://doi.org/10.1186/1472-6785-13-49
Himmler BT, Pellis SM, Kolb B (2013) Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neurosci Lett 556:42–45. https://doi.org/10.1016/j.neulet.2013.09.061
Himmler BT, Mychasiuk R, Nakahashi A, Himmler SM, Pellis SM, Kolb B (2017) Juvenile social experience and differential age-related changes in the dendritic morphologies of subareas of the prefrontal cortex in rats. Synapse 72:e22022. https://doi.org/10.1002/syn.22022
Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, Capitanio JP (2015) Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol 26:269–281. https://doi.org/10.1093/beheco/aru186
Holekamp KE, Smale L, Simpson HB, Holekamp NA (1984) Hormonal influences on natal dispersal in free-living Belding’s ground squirrels (Spermophilus beldingi). Horm Behav 18:465–483. https://doi.org/10.1016/0018-506X(84)90031-X
Jenkins SH, Eshelman BD (1984) Spermophilus beldingi. Mamm Species 221:1–8. https://doi.org/10.2307/3503911
Johnson MH (2001) Functional brain development in humans. Nat Rev Neurosci 2:475–483. https://doi.org/10.1038/35081509
Kapheim KM, Bernal SP, Smith AR, Nonacs P, Wcislo WT (2011) Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 65:1179–1190. https://doi.org/10.1007/s00265-010-1131-9
Kolb B, Gibb R (2011) Brain plasticity and behavior in the developing brain. J Can Acad Child Adolesc Psychiatry 20:256–276
Maestripieri D, Ross SR (2004) Sex differences in play among western lowland gorilla (Gorilla gorilla gorilla) infants: implications for adult behavior and social structure. Am J Physiol Anthropol 123:56–61. https://doi.org/10.1002/ajpa.10295
Marks KA, Vizconde DL, Gibson ES, Rodriguez JR, Nunes S (2017) Play behavior and responses to novel situations in juvenile ground squirrels. J Mammal 98:1202–1210. https://doi.org/10.1093/jmammal%2Fgyx049
Meder A (1990) Sex differences in the behaviour of immature captive lowland gorillas. Primates 31:51–63. https://doi.org/10.1007/BF02381029
Michener GR (1983) Kin identification, matriarchies, and the evolution of sociality in ground-dwelling sciurids. In: Eisenberg JF, Kleiman DG (eds) Advances in the study of mammalian behavior. American Society of Mammalogists, Stillwater, OK, pp 528–572
Morton ML, Gallup JS (1975) Reproductive cycle of the Belding ground squirrel (Spermophilus beldingi beldingi). Great Basin Nat 34:121–134
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407. https://doi.org/10.1016/S0169-5347(98)01472-4
Nolfo AP, Casetta G, Palagi E (2021) Play fighting in wild spotted hyenas: like a bridge over the troubled water of a hierarchical society. Anim Behav 180:363–373. https://doi.org/10.1016/j.anbehav.2021.07.012
Nunes S (2014) Juvenile social play and yearling behavior and reproductive success in female Belding’s ground squirrels. J Ethol 32:145–153. https://doi.org/10.1007/s10164-014-0403-7
Nunes S, Monroy Montemayor MP (2023) Multiple benefits of juvenile play: a ground squirrel’s perspective. Neurosci Biobehav Rev 147:105099. https://doi.org/10.1016/j.neubiorev.2023.105099
Nunes S, Zugger PA, Engh AL, Reinhart KO, Holekamp KE (1997) Why do female Belding’s ground squirrels disperse away from food resources? Behav Ecol Sociobiol 40:199–207. https://doi.org/10.1007/s002650050333
Nunes S, Muecke E-M, Anthony JA, Batterbee AS (1999) Endocrine and energetic mediation of play behavior in free-living ground squirrels. Horm Behav 36:153–165. https://doi.org/10.1006/hbeh.1999.1538
Nunes S, Muecke E-M, Ross HE, Bartholomew PA, Holekamp KE (2000) Food availability affects behavior but not circulating gonadal hormones in maternal Belding’s ground squirrels. Physiol Behav 71:447–455. https://doi.org/10.1016/s0031-9384(00)00366-8
Nunes S, Muecke E-M, Lancaster LT, Miller NA, Mueller MA, Muelhaus J, Castro L (2004) Functions and consequences of play behaviour in juvenile Belding's ground squirrels. Anim Behav 68:27–37. https://doi.org/10.1016/j.anbehav.2003.06.024
Nunes S, Weidenbach JN, Lafler MR, Dever JA (2015) Sibling relatedness and social play in juvenile ground squirrels. Behav Ecol Sociobiol 69:357–369. https://doi.org/10.1007/s00265-014-1848-y
Olioff M, Stewart J (1978) Sex differences in play behavior of prepubescent rats. Physiol Behav 20:113–115. https://doi.org/10.1016/0031-9384(78)90060-4
Palagi E (2018) Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behav Ecol Sociobiol 72:90. https://doi.org/10.1007/s00265-018-2506-6
Paukner A, Suomi SJ (2008) Sex difference in play behavior in juvenile tufted capuchin monkeys (Cebus apella). Primates 49:288–291. https://doi.org/10.1007/s10329-008-0095-0
Pederson JM, Glickman SE, Frank LG, Beach FA (1990) Sex differences in the play behavior of immature spotted hyenas, Crocuta crocuta. Horm Behav 24:403–420. https://doi.org/10.1016/0018-506x(90)90018-s
Pellis SM, Pellis VC (2017) What is play fighting and what is it good for? Learn Behav 45:355–366. https://doi.org/10.3758/s13420-017-0264-3
Pellis SM, Field EF, Smith LK, Pellis VC (1996) Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev 21:105–120. https://doi.org/10.1016/0149-7634(95)00060-7
Pellis SM, Pellis VC, Bell HC (2010) The function of play in the development of the social brain. Am J Play 2:278–296. https://doi.org/10.1016/j.neubiorev.2023.105037
Pellis SM, Pellis VC, Himmler BT (2014) How play makes for a more adaptable brain: a comparative and neural perspective. Am J Play 7:73–98
Pellis SM, Burghardt GM, Palagi E, Mangel M (2015) Modeling play: distinguishing between origins and current functions. Adapt Behav 23:331–339. https://doi.org/10.1177/1059712315596053
Pellis SM, Pellis VC, Pelletier A, Leca J-P (2019) Is play a behavior system, and, if so, what kind? Behav Process 160:1–9. https://doi.org/10.1016/j.beproc.2018.12.011
Pellis SM, Pellis VC, Ham JR, Stark RA (2023) Play fighting and the development of the social brain: the rat’s tale. Neurosci Biobehav Rev 145:105037
Petelle MB, McCoy DE, Alejandro V, Martin JGA, Blumstein DT (2013) Development of boldness and docility in yellow-bellied marmots. Anim Behav 86:1147–1154. https://doi.org/10.1016/j.anbehav.2013.09.016
Petelle MB, Martin JGA, Blumstein DT (2015) Heritability and genetic correlations of personality traits in a wild population of yellow-bellied marmots (Marmota flaviventris). J Evol Biol 28:1840–1848. https://doi.org/10.1111/jeb.12700
Petelle MB, Dang BN, Blumstein DT (2017) The effect of maternal glucocorticoid levels on juvenile docility in yellow-bellied marmots. Horm Behav 89:86–91. https://doi.org/10.1016/j.yhbeh.2016.12.014
Rasmussen JE, Belk MC (2017) Predation environment affects boldness temperament of neotropical livebearers. Ecol Evol 7:3059–3066. https://doi.org/10.1002/ece3.2886
Réale D, Gallant BY, Leblanc M, Festa-Bianchet M (2000) Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim Behav 60:589–597. https://doi.org/10.1006/anbe.2000.1530
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Réale D, Martin J, Coltman DW, Poissant J, Festa-Bianchet M (2009) Male personality, life-history strategies and reproductive success in a promiscuous mammal. J Evol Biol 22:1599–1607. https://doi.org/10.1111/j.1420-9101.2009.01781.x
Réale D, Dingemanse NJ, Kazem AJN, Wright J (2010) Evolutionary and ecological approaches to the study of personality. Phil Trans R Soc B 365:3937–3946. https://doi.org/10.1098/rstb.2010.0222
Rӧdel HG, Monclús R (2011) Long-term consequences of early development on personality traits: a study in European rabbits. Behav Ecol 22:1123–1130. https://doi.org/10.1093/beheco/arr100
Rӧdel HG, von Hulst D (2009) Features of the early juvenile development predict competitive performance in male European rabbits. Physiol Behav 97:495–502. https://doi.org/10.1016/j.physbeh.2009.04.005
Rӧdel HG, Bautista A, Roder M, Gilbert C, Hudson R (2017) Early development and the emergence of individual differences in behavior among littermates of wild rabbit pups. Physiol Behav 173:101–109. https://doi.org/10.1016/j.physbeh.2017.01.044
Sakai A, Sugiyama S (2018) Experience-dependent transcriptional regulation in juvenile brain development. Dev Growth Differ 60:473–482. https://doi.org/10.1111/dgd.12571
Sharpe LL (2005a) Frequency of social play does not affect dispersal partnerships in wild meerkats. Anim Behav 70:559–569. https://doi.org/10.1016/j.anbehav.2004.11.011
Sharpe LL (2005b) Play does not enhance social cohesion in a cooperative mammal. Anim Behav 70:551–558. https://doi.org/10.1016/j.anbehav.2004.08.025
Sharpe LL (2005c) Play fighting does not affect subsequent fighting success in wild meerkats. Anim Behav 69:1023–1029. https://doi.org/10.1016/j.anbehav.2004.07.013
Sharpe LL, Cherry MI (2003) Social play does not reduce aggression in wild meerkats. Anim Behav 66:989–997. https://doi.org/10.1006/anbe.2003.2275
Sih A, Bell A, Johnson CJ (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Sikes R, The Animal Care and Use Committee of the American Society of Mammalogists (2016) Guidelines of the American Society of Mammalogists for the use of wild animals in research and education. J Mammal 97:663–688. https://doi.org/10.1644/10-MAMM-F-355.1
Sinn DL, Gosling SD, Moltschaniwskyj NA (2008) Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim Behav 75:433–422. https://doi.org/10.1016/j.anbehav.2007.05.008
Skinner M, Brown S, Kumpan LT, Miller N (2022) Snake personality: differential effects of development and social experience. Behav Ecol Sociobiol 76:135. https://doi.org/10.1007/s00265-022-03227-0
Špinka M, Newberry RC, Bekoff M (2001) Mammalian play: training for the unexpected. Q Rev Biol 76:141–168. https://doi.org/10.1086/393866
Stark R, Pellis SM (2020) Male Long Evans rats reared with a Fischer-344 peer during the juvenile period show deficits in social competency: a role for play. Int J Play 9:76–91. https://doi.org/10.1080/21594937.2020.1720142
Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20:327–348. https://doi.org/10.1007/s11065-010-9148-4
Storm JJ, Lima SL (2010) Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am Nat 175:382–390. https://doi.org/10.1086/650443
Vetter SG, Brandstatter C, Macheiner M, Suchentrunk F, Gerritsmann H, Bieber C (2016) Shy is sometimes better: personality and juvenile body mass affect adult reproductive success in wild boars, Sus scrofa. Anim Behav 115:193–205. https://doi.org/10.1016/j.anbehav.2016.03.026
Wang T, Wang X, Garber PA, Sun B-H, Sun L, Xia D-P, Li J-H (2021) Sex-specific variation of social play in wild immature Tibetan macaques Macaca thibetana. Animals 11:805. https://doi.org/10.3390/ani11030805
Wauters LA, Mazzamuto MV, Santicchia F, Martinoli A, Preatoni DG, Lurz PWW, Bertolino S, Romeo C (2021) Personality traits, sex and food abundance shape space use in an arboreal mammal. Oecologia 196:65–76. https://doi.org/10.1007/s00442-021-04901-2
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behavior of the offspring. Progr Neurobiol 65:427–451. https://doi.org/10.1016/s0301-0082(01)00018-1
Whishaw IQ, Burke CJ, Pellis SM (2021) Does play shape hand use skill in rats? Exp Brain Res 235:1895–1909. https://doi.org/10.1007/s00221-021-06097-6
Wolff JO, Sherman PW (eds) (2007) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago
Shehan MI (2019) Play behavior and the development of boldness and caution in juvenile Belding’s ground squirrels (Urocitellus beldingi). MS thesis, University of San Francisco. https://repository.usfca.edu/thes/1207
Stark RA, Ramkumar R, Pellis SM (2021) Deficient play-deprived experiences in juvenile Long Evans rats reared with a Fischer 344 partner: a deficiency shared by both sexes. Int J Comp Psychol 34
Acknowledgements
We thank Nicole Thometz and Naupaka Zimmerman for their valuable insights throughout this work and assistance with statistical analyses. We further thank two anonymous reviewers for suggestions to improve the paper’s conceptual framework and statistical analyses. Naomi Logwood, Emma Gibson, Karen Marks, and Jennifer Rodriguez provided excellent assistance in the field.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study was supported by the Faculty Development Fund and Department of Biology at the University of San Francisco.
Author information
Authors and Affiliations
Contributions
JH-H and SN conceived and designed the study. All authors contributed to collection of data. JH-H, NL, and SN contributed to analysis of data. JH-H and SN wrote the first draft of the manuscript. All authors contributed to revision of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study followed guidelines for use of animals in field research published by the American Society of Mammalogists. Work for the study was conducted under permit SC-2008 from the California Department of Fish and Wildlife and permits LVD16018 and LVD17005 from the US Forest Service. Institutional approval for use of animals in the study was not required by the University of San Francisco.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by A. I Schulte-Hostedde
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 35 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hurst-Hopf, J.S., Monroy Montemayor, M.P., Leonardi, N.N. et al. Juvenile social play predicts docility in Belding’s ground squirrels. Behav Ecol Sociobiol 77, 62 (2023). https://doi.org/10.1007/s00265-023-03341-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03341-7