Abstract
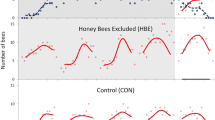
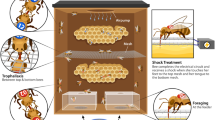
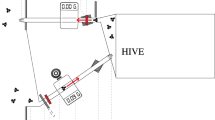
In a social species like the honey bee (Apis mellifera), changes in foraging strategy require shifts in several groups of specialized workers that are involved in collecting, storing, and processing food. In cases of extreme food shortage, honey bee colonies can switch to a high-risk, high-reward foraging tactic known as honey robbing, which involves stealing mature honey from other colonies. Colonies engaged in honey robbing show a corresponding increase in defensive behaviors displayed by specialist guard bees, presumably because the conditions that provoke robbing also increase the risk of colony invasion. Previous studies suggest aggressive behaviors displayed by robbing forager nestmates modulate guard defensiveness. In the current study, we evaluated which aspects of the robbing experience likely alter forager aggression, and in turn, guard defensiveness. We trained colonies to visit feeders containing either raw honey or a sucrose solution and examined whether food type, experience of conflict at the feeder, or other abiotic cues that reflect the time of the season best explain variation in guard defensiveness. We found little evidence that food type influences forager interactions with guards. Rather, conflict at the feeder is the best predictor of increased aggressive interactions, even when accounting for the effects of seasonal change. Thus, intraspecific conflict at the food resource during robbing may drive shifts in individual forager aggression, activating guard defensiveness as one component of a syndrome of colony-level changes required to accommodate the robbing foraging tactic.
Significance statement
Honey bees possess an extreme foraging tactic that they employ under conditions of resource scarcity. This tactic, honey robbing, requires coordinated changes among worker bees to accommodate enhanced food collection, processing, and storing, as well as nest defense. In a previous study, we showed that robbing foragers show unusually high aggression, and that this shift may trigger greater defensiveness from nestmate guards once foragers return home. Here, we explored the cues that coordinate the change in defensive effort from guards and find that forager conflict at the food resource is a strong predictor of guard defensiveness. These results suggest that guards use behavioral cues from their own foragers to estimate their risk of attack and increase their defensiveness accordingly.




Similar content being viewed by others
Data availability
Raw data is publicly available: https://figshare.com/articles/dataset/Napieretal_RawData_xlsx/21953345
References
Alaux C, Robinson GE (2007) Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J Chem Ecol 33:1346–1350
Alaux C, Sinha S, Hasadsri L, Hunt GJ, Guzman-Novoa E, Degrandi-Hoffman G, Uribe-Rubio JL, Southey BR, Rodriguez-Zas S, Robinson GE (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. Proceed National Acad Sci USA 106:15400–15405
Aljohar HI, Maher HM, Albaqami J, Al-Mehaizie M, Orfali R, Orfali R, Alrubia S (2018) Physical and chemical screening of honey samples available in the Saudi market: an important aspect in the authentication process and quality assessment. Saudi Pharm J 26:932–942
Balderrama NM, de Almeida LOB, Nunez JA (1992) Metabolic rate during foraging in the honeybee. J Comp Physiol B 162:440–447
Barnett CA, Bateson M, Rowe C (2007) State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav Ecol 18:645–651
Bell HC, Hsiung K, Pasberg P, Broccard FD, Nieh JC (2021) Responsiveness to inhibitory signals changes as a function of colony size in honeybees (Apis mellifera). J R Soc Interface 18:20210570
Berger D, Gotthard K (2008) Time stress, predation risk and diurnal–nocturnal foraging trade-offs in larval prey. Behav Ecol Sociobiol 62:1655–1663
Blatt J, Roces F (2001) Haemolymph sugar levels in foraging honey bees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J Exp Biol 204:2709–2716
Bloxham L, Bateson M, Bedford T, Brilot B, Nettle D (2014) The memory of hunger: developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris. Anim Behav 91:33–40
Borofsky T, Barranca VJ, Zhou R, von Trentini D, Broadrup RL, Mayack C (2020) Hive minded: like neurons, honey bees collectively integrate negative feedback to regulate decisions. Anim Behav 168:33–44
Breed MD (1983) Nestmate recognition in honey bees. Anim Behav 31:86–91
Breed MD, Garry MF, Pearce AN, Hibbard BE, Bjostad LB, Page RE (1995) The role of wax comb in honey bee nestmate recognition. Anim Behav 50:489–496
Breed MD, Robinson GE, Page RE (1990) Division of labor during honey bee colony defense. Behav Ecol Sociobiol 27:395–401
Breed MD, Williams KR, Fewell JH (1988) Comb wax mediates the acquisition of nest-mate recognition cues in honey bees. Proc Natl Acad Sci 85:8766–8769
Carlesso D, Smargiassi S, Pasquini E, Bertelli G, Baracchi D (2021) Nectar non-protein amino acids (NPAAs) do not change nectar palatability but enhance learning and memory in honey bees. Sci Rep 11:11721
Charalabidis A, Dechaume-Moncharmont FX, Petit S, Bohan DA (2017) Risk of predation makes foragers less choosy about their food. PLoS One 12:e0187167
Couvillon MJ, Robinson EJH, Atkinson B, Child L, Dent KR, Ratnieks FLW (2008) En garde: rapid shifts in honeybee, Apis mellifera, guarding behaviour are triggered by onslaught of conspecific intruders. Anim Behav 76:1653–1658
Couvillon MJ, Schurch R, Ratnieks FL (2014a) Dancing bees communicate a foraging preference for rural lands in high-level agri-environment schemes. Curr Biol 24:1212–1215
Couvillon MJ, Schurch R, Ratnieks FL (2014b) Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS One 9:e93495
Doner LW (1977) The sugars of honey - a review. J Sci Food Agric 28:443–456
Downs SG, Ratnieks FL (2000) Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav Ecol 11:326–333
Downs SG, Ratnieks FL, Jefferies SL, Rigby HE (2000) The role of floral oils in the nestmate recognition system of honey bees (Apis mellifera L.). Apidologie 31:357–365
Downs SG, Ratnieks FLW, Badcock NS, Mynott A (2001) Honeybee guards do not use food-derived odors to recognize non-nest mates: a test of the Odor Convergence hypothesis. Behav Ecol 12:47–50
Eiri DM, Nieh JC (2016) A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J Exp Biol 219:2081
Free JB (1954) The behaviour of robber honeybees. Behaviour 7:233–240
Gao J, Zhao G, Yu Y, Liu F (2010) High concentration of nectar quercetin enhances worker resistance to queen’s signals in bees. J Chem Ecol 36:1241–1243
Garbuzov M, Balfour NJ, Shackleton K, Al Toufailia H, Scandian L, Ratnieks FLW (2020) Multiple methods of assessing nectar foraging conditions indicate peak foraging difficulty in late season. Insect Conserv Divers 13(6):532–542. https://doi.org/10.1111/icad.12420
Grume GJ, Biedenbender SP, Rittschof CC (2021) Honey robbing causes coordinated changes in foraging and nest defence in the honey bee, Apis mellifera. Anim Behav 173:53–65
Guzman-Novoa E, Page RE (1994) Genetic dominance and worker interactions affect honeybee colony defense. Behav Ecol 5:91–97
Harrison JW, Palmer JH, Rittschof CC (2019) Altering social cue perception impacts honey bee aggression with minimal impacts on aggression-related brain gene expression. Sci Rep 9:14642
Herb BR, Shook MS, Fields CJ, Robinson GE (2018) Defense against territorial intrusion is associated with DNA methylation changes in the honey bee brain. BMC Genomics 19:216
Higginson AD, Barnard CJ (2004) Accumulating wing damage affects foraging decisions in honeybees (Apis mellifera). Ecological Entomology 29:52–59
Lau CW, Nieh JC (2010) Honey bee stop-signaling production: temporal distribution and effect of feeder crowding. Apidologie 41:87–95
Li-Byarlay H, Rittschof CC, Massey JH, Pittendrigh BR, Robinson GE (2014) Socially responsive effects of brain oxidative metabolism on aggression. Proc Natl Acad Sci U S A 111:12533–12537
Mao W, Schuler MA, Berenbaum MR (2013) Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc Natl Acad Sci U S A 110:8842–8846
Moore AJ, Breed MD, Moor MJ (1987) The guard honey bee: ontogeny and behavioural variability of workers performing a specialized task. Anim Behav 35:1159–1167
Mujagic S, Erber J (2009) Sucrose acceptance, discrimination and proboscis responses of honey bees (Apis mellifera L.) in the field and the laboratory. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195:325–339
Nieh JC (2010) A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr Biol 20:310–315
Njoroge TM, Calla B, Berenbaum MR, Stone CM (2021) Specific phytochemicals in floral nectar up-regulate genes involved in longevity regulation and xenobiotic metabolism, extending mosquito life span. Ecol Evol 11:8363–8380
Núñez JA, Giurfa M (1996) Motivation and regulation of honey bee foraging. Bee World 77:182–196
Pamminger T, Becker R, Himmelreich S, Schneider CW, Bergtold M (2019) The nectar report: quantitative review of nectar sugar concentrations offered by bee visited flowers in agricultural and non-agricultural landscapes. PeerJ 7:e6329
Pankiw T, Waddington KD, Page RE Jr (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): influence of genotype, feeding, and foraging experience. J Comp Physiol A 187:293–301
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS One 14:e0218392
Prado A, Requier F, Crauser D, Le Conte Y, Bretagnolle V, Alaux C (2020) Honeybee lifespan: the critical role of pre-foraging stage. Royal Soc Open Sci 7(11). https://doi.org/10.1098/rsos.200998
Preston SR, Palmer JH, Harrison JW, Carr HM, Rittschof CC (2019) The impacts of maternal stress on worker phenotypes in the honey bee. Apidologie 50:704–719
Reader T, MacLeod I, Elliott PT, Robinson OJ, Manica A (2005) Inter-order interactions between flower-visiting insects: foraging bees avoid flowers previously visited by hoverflies. J Insect Behavior 18:51–57
Ribbands CR, Kalmus H, Nixon HL (1952) New evidence of communication in the honeybee colony. Nature 170:438–440
Richard FJ, Holt HL, Grozinger CM (2012) Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera). BMC Genomics 13:1–17
Rittschof CC (2017) Sequential social experiences interact to modulate aggression but not brain gene expression in the honey bee (Apis mellifera). Front Zool 14:1–10
Rittschof CC, Bukhari SA, Sloofman LG, Troy JM, Caetano-Anolles D, Cash-Ahmed A, Kent M, Lu X, Sanogo YO, Weisner PA, Zhang H, Bell AM, Ma J, Sinha S, Robinson GE, Stubbs L (2014) Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee. Proc Natl Acad Sci U S A 111:17929–17934
Rittschof CC, Coombs CB, Frazier M, Grozinger CM, Robinson GE (2015) Early-life experience affects honey bee aggression and resilience to immune challenge. Sci Rep 5:15572
Rittschof CC, Nieh JC (2021) Honey robbing: could human alterations to the environment change a rare foraging tactic into a maladaptive behavior? Curr Opin Insect Sci 45:54–90
Rittschof CC, Robinson GE (2013) Manipulation of colony environment modulates honey bee aggression and brain gene expression. Genes Brain Behav 12:802–811
Rivera-Marchand B, Giray T, Guzmán-Novoa E (2008) The cost of defense in social insects: insights from the honey bee. Entomol Exp Appl 129:1–10
Roitberg BD, Keiser S, Hoffmeister T (2010) State-dependent attacks in a mosquito. Physiol Entomol 35:46–51
Sasaki T, Pratt SC (2018) The psychology of superorganisms: collective decision making by insect societies. Annu Rev Entomol 63:259–275
Schmid-Hempel P, Kacelnik A, Houston AI (1985) Honeybees maximize efficiency by not filling their crop. Behav Ecol Sociobiol 17:61–66
Seeley TD (1986) Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav Ecol Sociobiol 19:343–354
Seeley TD (1989) Social foraging in honey bees: how nectar foragers assess their colony’s nutritional status. Behav Ecol Sociobiol 24:181–199
Seeley TD (1994) Honey bee foragers as sensory units of their colonies. Behav Ecol Sociobiol 34:51–62
Seeley TD (1997) Honey bee colonies are group-level adaptive units. Am Nat 150:s22–s41
Seeley TD, Tovey CA (1994) Why search time to find a food-storer bee accruately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Anim Behav 47:311–316
Shafir S, Waite T, Smith B (2002) Context-dependent violations of rational choice in honeybees ( Apis mellifera ) and gray jays ( Perisoreus canadensis ). Behav Ecol Sociobiol 51:180–187
Shpigler HY, Saul MC, Murdoch EE, Cash-Ahmed AC, Seward CH, Sloofman L, Chandrasekaran S, Sinha S, Stubbs LJ, Robinson GE (2017) Behavioral, transcriptomic and epigenetic responses to social challenge in honey bees. Genes Brain Behav 16:579–591
Simcock NK, Gray H, Bouchebti S, Wright GA (2018) Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J Insect Physiol 106:71–77
Skelhorn J, Rowe C (2007) Predators’ toxin burdens influence their strategic decisions to eat toxic prey. Curr Biol 17:1479–1483
van Alphen JJM, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
Van Nest BN, Moore D (2018) How to train a honey bee. J Undergrad Neurosci Educ 17:T1–T11
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464
Waller GD (1972) Evaluating responses of honey bees to sugar solutions using an artificial flower feeder. Ann Entomol Soc Am 65:857–862
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Massachusetts
Acknowledgements
We thank Jimmy Harrison and Anna Foose for help with experimental set-up and honey bee colony management.
Funding
This work was funded by a University of Kentucky Office of Undergraduate Research Summer Research Fellowship (to T.C.N.), the National Science Foundation (to C.C.R., IOS-2045901), the National Institute of Food and Agriculture Hatch Program (to C.C.R., 1012993), the Kentucky Agriculture Development Fund (to C.C.R., A2019-0266), and the Foundation for Food and Agriculture Research (to C.C.R., 549049).
Author information
Authors and Affiliations
Contributions
T.C.N, R.R.W., and C.C.R conceptualized and designed the study. T.C.N., R.R.W., and C.W.K. collected data. T.C.N. drafted an original version of the manuscript. C.C.R. performed the data analysis and drafted the final version of the manuscript. R.R.W. and C.W.K. contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was designed so that there was no known mortality impacts on honey bee individuals or colonies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by D. Naug
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Napier, T.C., Westwick, R.R., Kane, C.W. et al. Evaluating the cues that coordinate a shift towards the robbing foraging tactic in the honey bee (Apis mellifera). Behav Ecol Sociobiol 77, 46 (2023). https://doi.org/10.1007/s00265-023-03321-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03321-x