Abstract
Developmental environments play a significant role in shaping animal phenotype, including behavior. Within a species, individuals often differ in behavior in a consistent and repeatable way (i.e., demonstrate animal personality). This consistency in behavior can be affected by differences in conditions experienced early in life. It is, however, unclear whether effects of developmental environments on animal personality are driven by changes in within- or between-individual variation. To investigate this, we measured activity, exploration, sociability, and boldness in adult male southern rainforest sunskinks, Lampropholis similis, incubated at either 23 °C or 26 °C, and compared behavioral phenotypes between these incubation treatments. We also compared the behavior of these incubation groups to a cohort of wild-caught skinks to determine whether rearing in captivity also affected the personality of the lizards. Skinks that had been incubated at a higher temperature were more explorative and demonstrated personality in a larger suite of traits compared to lizards incubated at a lower temperature or caught in the wild. These differences among developmental environment were primarily driven by within-individual variation, which tended to be higher among the high incubation treatment. We also found no evidence for a behavioral syndrome in either captive- or wild-reared skinks. Our results suggest the potential for greater behavioral plasticity in skinks incubated at a higher temperature, which may enable them to cope with environmental change, such as climate warming, in the short term. Overall, we show that effects of developmental environment are complex and play a pivotal role in shaping animal personality.
Significance statement
Experiences during development are expected to influence how animals develop, including their behavior. We tested early environment effects on behavior in adult southern rainforest sunskinks by comparing lizards incubated at different temperatures as well as comparing those reared in the wild with those reared in captive environments. We found that lizards incubated at the higher temperature were more exploratory. Furthermore, both incubation temperature and captivity/wild-rearing had pronounced effects on the consistency of behavior—in different directions for different traits—demonstrating developmental environments have strong effects on animal personality. Such changes in behavioral traits likely have flow-on effects for the animal’s fitness and biotic interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many species, individuals exhibit relatively consistent differences in behavior, which has been termed “animal personality” (Sih et al. 2004; Réale et al. 2007; Bell et al. 2009; Moiron et al. 2020). Personality traits have important roles in evolutionary and ecological processes, because they can correlate with fitness and can impact interactions within and among species (Réale et al. 2007; Smith and Blumstein 2008; Bolnick et al. 2011; Wolf and Weissing 2012). Understanding what causes and maintains variation in personality among individuals is therefore of considerable interest, particularly as environments are changing rapidly as a result of human influence.
Environments experienced during development can have significant and long-lasting impacts on phenotype and fitness (West-Eberhard 2003) and may be one way in which differences in personality emerge. Animal personality is quantified by repeatability, which is the ratio of between-individual variance (VID) to phenotypic variance (VID + within-individual variance [VR]). Previous studies have found impacts of developmental environments on repeatability in behavior. For example, agile frog tadpoles, Rana dalmatina, only had significant repeatability in risk-taking behavior when reared in an environment containing predator scent (Urszan et al. 2015). Similarly, DiRienzo et al. (2015) found that early exposure to pathogens resulted in lower repeatability in some boldness measures in field crickets, Gryllus integer. Changes in repeatability can be due to shifts in between- or within-individual variation, increasing when between-individual variance increases and decreasing when within-individual variance increases. For example, crickets, G. bimaculatus, reared at a high temperature had increased both within- and between-individual variation (Niemelä et al. 2019. Together, these studies demonstrate a clear role for early environments in affecting personality. However, it is largely unknown whether this is driven by changes in within- or between-individual variation, despite increasing interest in quantifying these two components of repeatability.
Like most phenotypic traits, behaviors are not expressed in isolation but are correlated with a range of traits, including other behaviors (Sih et al. 2004). Developmental environment effects may therefore extend to, or be constrained by, the stability of these correlations. Suites of behaviors correlated across time and contexts are termed “behavioral syndromes” (Sih et al. 2004) and are found in many species, including spiders (Johnson and Sih 2007), fish (Bell and Sih 2007; Norton et al. 2011), lizards (Michelangeli et al. 2019), birds (Carere et al. 2005; Dingemanse et al. 2012), and mammals (Dingemanse and Réale 2005). Whether these correlations constrain the effects of developmental environments may depend on whether they reflect fundamental genetic constraints or arise due to independent but correlated plastic responses to the environment. It is still unclear which is the predominant mechanism (Bell 2005), with evidence for both hypotheses. For example, work on Drosophila (Sokolowski 2001), zebrafish, Danio rerio (Norton et al. 2011), and bighorn ewes, Ovis canadensis (Dingemanse and Réale 2005), support a strong genetic basis for behavioral syndromes. In contrast, support for environmental effects and instability within short time periods has been seen in agile frog tadpoles, R. dalmatina (Urszan et al. 2015) and three-spined sticklebacks, Gasterosteus aculeatus (Bell and Sih 2007; Dingemanse et al. 2007). If the latter is the case, it would suggest that developmental environments could shape these between-individual correlations, although our understanding of how these early environments affect behavioral syndromes is still limited (Han and Dingemanse 2015; Royauté and Dochtermann 2017).
In our study, we investigated whether developmental environment affects animal personality using a small lizard species. Developmental temperature plays an important role in the development of many species, particularly for taxa such as reptiles, where many traits are affected by incubation temperature (Noble et al. 2018). These effects may be driven by different costs of development (Marshall et al. 2020) or expression of hormones, such as in leopard geckos, Eublepharis macularius (Rhen et al. 2005), which can then affect phenotypic plasticity (Dufty et al. 2002). Lizards from the Lampropholis genus are regularly used in studies investigating animal personality (e.g., Michelangeli et al. 2016a, 2018; Goulet et al. 2021) as well as effects of incubation temperature on physiology and behavior (e.g., Downes and Shine 1999; Llewelyn et al. 2018; Kar et al. 2022). Accordingly, we used a species of Lampropholis skink to investigate the impacts of incubation temperature on animal personality. Southern rainforest sunskinks, L. similis (also known as southern Wet Tropics sunskinks), are small diurnal skinks (adult snout-vent length (SVL) ~ 45 mm) endemic to the wet tropics of Queensland’s north-east (Wilson and Swan 2021). In this species, incubation temperature was found to affect dessication rate and short-term critical thermal minima (Llewelyn et al. 2018), but effects of incubation temperature on behavior have not yet been investigated. Furthermore, while no behavioral syndrome has yet been found in captive-reared southern rainforest sunskinks (Goulet et al. 2021), previous studies have suggested that natural environments may be necessary to generate and maintain repeatability in, and correlations between, behavioral traits (Wilson et al. 1994; Urszan et al. 2015). As such, a secondary aim of our study was to investigate if skinks that had developed in captivity differed in animal personality from those that had developed in the wild.
We first compared four behavioral traits (activity, exploration, sociability, and boldness) of lizards incubated at 23 °C and 26 °C to examine how incubation temperature affected mean behavior, repeatability, within- and between-individual variation, and correlations among traits. We predicted that mean behavior would differ among treatments, and, given that Niemelä et al. 2019) found higher within- and between-individual variation in crickets developing under hotter temperatures, we predicted rainforest sunskinks incubated at the hotter temperature would also have higher estimates for both variance components. We then examined whether there were any differences in the same behavioral measures between the behavior of both incubation temperature groups to that of their fathers, who had been captured from a wild population. We focused on only males, and hence fathers rather than mothers, as there is evidence for limited sex-specific personality in Lampropholis skinks (Michelangeli et al. 2016a; Goulet et al. 2021), as well as to exclude reproductive state as a confounding factor. Repeatability was expected to be higher in the wild-reared skinks, which may be due to past exposure to factors such as predation, as in agile frog tadpoles (Urszan et al. 2015), or due to greater varation in natural environments allowing populations to express greater between-individual variation (Bell et al. 2009).
Methods
Capture, housing, and breeding
Adult skinks were hand-captured from a population in Hervey Range (19.362° S, 146.4767° E), located at the southern end of the Wet Tropics of north-east Queensland, Australia, between June 2013 and January 2014. Hand-capture of Lampropholis skink species samples a range of behavioral types (Michelangeli et al. 2016b), thus mitigating the likelihood of capture bias. Skinks were transported to James Cook University in Townsville where they were housed individually in plastic tubs (340 × 120 × 160 mm) for 6 months before mating pairs were established as part of another experiment (Llewelyn et al. 2017, 2018). Mating tubs were checked daily for eggs, and, if no fertile eggs were laid within 2 months, mating partners were swapped. Individual eggs were placed into 84-mL air-tight containers containing moist vermiculite (50:50 vermiculite to water by weight) and incubated at either 23 °C or 26 °C. The incubators were checked daily and, once hatched, the skinks were housed individually and given a unique toe clip for identification. Skinks were housed in small groups when they reached adult size (> 0.75 g). Hatching took place over the course of approximately one year, between January 2014 and March 2015. The parents were also transferred to small, single sex groups following breeding, and the males later formed the wild-reared group in our study (hereafter fathers). In 2015, the fathers, low incubation, and high incubation groups were transported to Monash University, Clayton, and housed in same-sex groups of six in plastic containers (300 × 230 × 370 mm) in temperature-controlled rooms (~ 22 °C). The containers were lined with newspaper and contained moist Sphagnum moss and a terracotta saucer with a heat lamp to create a basking site. Lights were on between 07:00 and 19:00, following a 12:12 day:night cycle, and were supplemented with ultraviolet lighting. Water was provided ad libitum, and the skinks were fed live crickets, Acheta domesticus, dusted in calcium and vitamin powders three times per week.
Experimental design
Over the 2016/2017 Austral summer, adult male skinks, including the fathers (n = 21) and those from the low incubation (23 °C: n = 11) and high incubation (26 °C: n = 10) treatments, underwent separate assays to measure activity, exploration, sociability, and boldness behaviors. At the beginning of the experiments, the youngest lab-born skink was 781 days old. As Lampropholis lizards mature after 1 year under optimal conditions, all our experimental animals were therefore mature adults. The individuals selected for the study from the two incubation temperature treatments were unrelated, i.e., no siblings or half-siblings.
Assays were carried out in a fixed order to reduce possible carry over effects, with those expected to have the greatest influence on subsequent behavior completed last (Bell 2012). Each behavior was measured three times before moving on to the next. Activity was measured first, followed by exploration, sociability, and then boldness. Each replicate was conducted at least four days apart to further minimize potential carryover effects (Bell 2012), with a minimum of four days also between different behavioral assays. We did not provide food in the 24 h prior to testing to standardize digestion levels, as feeding is known to alter the behavior of Lampropholis skinks (Shine 2003). To assess possible body size effects on behavior, snout-vent length (SVL; mm) and mass (g) were measured before experimentation.
Trials were carried out in opaque plastic containers (~ 55 × 32 × 24 cm) in temperature-controlled rooms (~ 22 °C). We used a video camera to record trials, rather than direct observation, to avoid observer-induced disturbance. Focal skinks were individually acclimated to the experimental area for 10 min under a clear plastic container, which was removed at the start of each trial. To prevent scent contamination between trials, following each test the equipment was washed with scentless detergent and thoroughly dried (Downes and Shine 1998). Each behavioral assay was conducted three times per individual to measure repeatability (Nakagawa and Schielzeth 2010; Wolak et al. 2012). At the conclusion of all assays, behaviors were scored from the video recordings using the program JWatcher (Blumstein et al. 2006). Scoring was performed blind to incubation temperature treatment, but not to captive or wild origin.
Activity
To measure activity, we observed the distance individual skinks moved in an open-field arena marked with 20 equal grid squares. We recorded a count of the number of times a focal skink crossed from one square into another over 45 min (Chapple et al. 2011; Michelangeli et al. 2016b).
Exploration
To measure propensity to explore, we observed individuals’ tendency to traverse an obstacle to find an essential resource, a basking site (Chapple et al. 2011; Goulet et al. 2017). We created an obstacle using an opaque barrier (10 cm high) with a polyvinyl chloride (PVC) tube (4-cm diameter, 10 cm in length) running through the middle of the barrier, located approximately 1.5 cm from the ground level. The barrier spanned the width of an arena and divided it into two compartments; one which contained a basking site constructed from a terracotta saucer and heat lamp, and the other where skinks began the experiment. The only way that skinks could reach the basking site was by finding their way through the PVC tube. We measured the time spent inspecting the barrier (a novel object as it is not present in skinks’ home cages) and latency to reach the basking site within 45 min.
Sociability
To determine sociability, we presented individual skinks with a choice between basking with conspecifics or alone (Chapple et al. 2011; Michelangeli et al. 2016a, b; Goulet et al. 2017). Two basking sites were placed at either end of the arena, each divided by clear Perspex™ barriers that spanned the width of the arena and enabled basking on both sides of the barriers. This created three areas in the arena; one containing three male southern rainforest sunskinks that had been born in captivity but were not part of any experimental treatment to simulate a social group, a central area containing the focal skink, and a third that remained empty. The central area was then further divided into three using black marker with zones designated as “social,” “no-choice,” and “asocial” with increasing distance from the area containing the social group. Both the “social” and “asocial’ zones contained basking sites as they were adjacent to the Perspex™ barriers. Heat lamps raised the temperatures at the basking sites to ~ 35 °C, prompting the focal skink to select a basking site either in the “social” or “asocial” zone. We measured the time spent basking in the ‘social’ zone over a 45 min period.
Boldness
We measured boldness by observing the latency of individual skinks to emerge from shelter and subsequently bask after a simulated predator attack (Michelangeli et al. 2016b). The arena contained a basking site at one end and shelter at the other. We simulated predator attacks by using a small paintbrush to tap the skinks at the base of their tail until they fled into the shelter. We recorded their latency to emerge from shelter, with a maximum time allowed of 25 min and then the amount of time basking over an additional 10 min after emergence. For skinks that did not emerge, an emergence time of 25 min and a basking score of 0 min was given.
Statistical analysis
Data were analyzed using the program R (R Development Core Team 2016), with statistical significance set to α = 0.05. We tested for age effects on both incubation temperature groups, as they had hatched over the course of approximately 14 months. As there was no significant effect of age on behavior (p > 0.200), we excluded age as a covariate in our models.
To determine whether incubation temperature affected mean activity, exploration, sociability, or boldness, and to compare these to the wild-reared fathers, we used separate mixed-effect models for each behavior. Latency (s) to emerge from shelter (boldness) and to reach the goal compartment (exploration) were both analyzed using Cox mixed-effects models (Therneau 2020), activity was analyzed with a generalized linear mixed-effects model with a Poisson distribution, and all other behavioral measures were analyzed with linear mixed models fitted with Gaussian error distributions using the lme4 package (Bates et al. 2015). Time basking in the social zone and time basking after emerging from shelter were both square root transformed to meet assumptions. Models included lizard ID as a random effect to control for repeated measures, and mass (scaled), time of day (scaled), trial number, and treatment (fathers, high incubation, low incubation) were included as fixed effects. We used a combination of Akaike information criteria (AIC) and likelihood ratio tests (LRT) to examine whether interactions among fixed effects or, as there were some father and son pairs, including family ID as a random effect, improved model fit. Interactions that improved model fit were retained in the final model, but family ID did not improve the fit for any behavioral variable and so was excluded from further analysis. Furthermore, we examined whether SVL was a better predictor of body size effects on behavior, either as a substitution for, or in addition to, mass using AIC and LRTs. Mass was the better predictor, or had equal support, compared to SVL (Table S1), and so we controlled for body size using mass. When factors with more than three levels were significant, we used the emmeans R package (Lenth 2020) to perform post hoc pairwise comparisons among groups.
Repeatability estimates (R) and variance components (VID and VR) were obtained from Bayesian univariate mixed-effects models using the brms package (Burkner 2017), with repeatability estimated as VID/(VID + VR). Models were specified with weakly informative priors and ran for 10,000 iterations (3000 warmup) with the same error distributions, transformations, and fixed and random effect structure as above. We calculated repeatability and variance components fitted separately for each group of skinks, and so treatment was not included as a fixed effect in these models. Trace plots were visually inspected to ensure convergence and proper model mixing. To estimate the effect size of treatment on the variance components and repeatability estimates, we calculated the pairwise difference between posterior distributions and used this to estimate the mean difference and 95% credible intervals (CrI) (Royauté et al. 2015; Royauté and Dochtermann 2017). Repeatable behaviors were then included in separate multivariate Bayesian mixed-effect models for each group to test for the presence of behavioral syndromes, with the same priors, error distributions, transformations, and fixed and random effects structure. Inference for all tests was based on means and where CrIs did not overlap with zero.
Results
Mean behavioral differences
Activity was affected by the interaction between developmental environment and trial number (Table 1; Fig. 1a). Mean activity did not differ among developmental environments (p > 0.325), but there were differences among groups in how activity changed across trials. All groups decreased activity from trial one to trial two, but only the wild-caught fathers further decreased activity in the third compared to second trial (Table S2; Fig. 1a). Mass and time of day also had a positive relationship with activity (Table 1).
Effects of developmental environment (wild: gray; high incubation: yellow; and low incubation: teal) on activity (a) across trials 1, 2, and 3; exploration (b time to reach the goal compartment; c time inspecting the barrier); sociability (d); and boldness behaviors (e time to emerge from shelter; f time basking after emerging from shelter) in rainforest sunskinks. Boxplots show medians, interquartile ranges (IQR), and whiskers the 1.5 × IQR
We also found significant effects of developmental environment on exploratory behavior (Table 1; Fig. 1b, c). Skinks incubated in the high temperature treatment were more explorative than both the wild-caught fathers and those incubated in the low temperature treatment, as indicated by a higher proportion of individuals reaching the goal compartment (Table S2; Fig. 1b) and a greater amount of time inspecting the barrier (Table S2; Fig. 1c). In contrast, skinks incubated at the low temperature did not differ in exploratory behavior from the wild-caught fathers (Table S2; Fig. 1b, c). Trial number significantly affected time inspecting the barrier, but not latency to reach the goal compartment (Table 1). Skinks from all developmental environments spent less time inspecting the barrier in trial two compared to trial one (− 32.15 ± 12.9 SE, p = 0.039), but time spent inspecting the barrier did not significantly differ between trials two and three (− 26.12 ± 12.8, p = 0.110), or between trials one and three (6.03 ± 13.0, p = 0.888). Skinks were also more explorative later in the day and when they were heavier (Table 1).
Sociability was not significantly affected by developmental environment and was the only behavior not significantly affected by mass (Table 1; Fig. 1d). We also found some limited effects of developmental environment on boldness (Table 1). Developmental environment did not have a significant effect on latency to emerge from shelter (Table 1; Fig. 1e), but did have a significant effect on time basking after emerging from shelter in the main model (Table 1). However, post hoc pairwise comparisons revealed no significant differences among incubation treatments, as well as non-significant decrease in basking behavior in the wild-caught compared to lab-reared skinks (Table S2; Fig. 1f). Mass had a positive relationship with boldness, decreasing latency to emerge from shelter and increasing time basking after emerging from shelter (Table 1).
Repeatability and variation
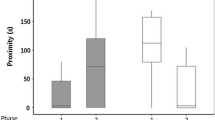
Behavioral repeatability differed among developmental environments and behaviors. Only two behaviors were repeatable for skinks from all three developmental environments: activity and time inspecting the barrier (Table 2). For activity, repeatability was higher in the low incubation treatment compared to both the high incubation treatment and wild-caught fathers (Table S3; Fig. 2a), while for time inspecting the barrier the low incubation treatment had higher repeatability compared to only the high incubation treatment (Table S3; Fig. 2b). In contrast to these behaviors, latency to emerge from shelter and sociability were only repeatable in skinks incubated at the high temperature, and time basking after emerging from shelter was repeatable for both the low incubation treatment and wild-caught fathers (Table 2). The low incubation treatment had a higher repeatability estimate for basking time compared to the fathers (Table S3; Fig. 2f). No developmental treatment group was repeatable for latency to emerge from shelter (Table 2).
Pairwise differences in behavioral repeatability estimates between southern rainforest sunskinks that had developed in the wild (fathers) or in captivity at a low or high incubation temperature. Differences are shown for activity (a), exploration (b time inspecting barrier; c latency to reach goal), sociability (d), and boldness (e latency to emerge from shelter; f basking after emerging) behavior. Circles represent mean difference between groups, and vertical lines show 95% credibility intervals. Repeatability estimates were derived from posterior distributions of Bayesian mixed-effects models
Developmental environment only affected between-individual variation in three behaviors (Table S3). When comparing incubation treatments, skinks incubated at a low temperature had higher between-individual variation for activity and time basking after emerging from shelter (Table S3; Fig. 3a, f), while between-individual variation was higher in the high incubation treatment for sociability (Table S3; Fig. 3d). There were a greater number of differences in within-individual variation among incubation treatments (Table S3). Within-individual variation was more frequently higher in the high incubation treatment, which was the case for activity, time inspecting the barrier, and both boldness behaviors, latency to emerge from shelter and time basking after emerging from shelter (Fig. 3a, b, e, f). However, for latency to reach the goal and sociability, within-individual variation was higher in the low incubation treatment (Fig. 3c, d).
Pairwise differences in between-individual variation (VID; black circles) and within-individual variation (VR; gray triangles) of southern rainforest sunskinks that developed in the wild (fathers) or in captivity at a low or high incubation temperature. Differences are shown for activity (a), exploration (b time inspecting barrier; c latency to reach goal), sociability (d), and boldness (e latency to emerge from shelter; f basking after emerging) behavior. Values represent differences in mean variation between groups and vertical lines show 95% credibility intervals. Estimates were derived from posterior distributions of Bayesian mixed-effects models
Wild- and captive-reared skinks also differed in between-individual variation. This tended to be lower in the wild-reared fathers, although whether the difference was significant depended on incubation treatment (Table S3; Fig. 3a–f). The only exception was for time basking after emerging from shelter, where the fathers had higher between-individual variation compared to the high incubation treatment (Table S3; Fig. 3f). Similar to differences among incubation treatments, there were a greater number of differences in within-individual variation between captive- and wild-reared skinks. Within-individual variation was also lower in the fathers for most behaviors when compared to both incubation treatments (Table S3; Fig. 3a–f). There was also an exception where within-individual variation was higher for the high incubation treatment compared to the fathers for latency to reach the goal compartment (Fig. 3c).
Behavioral syndromes
The treatments differed in which behaviors were repeatable (Table 3; Fig. 2), and so the models examining between-individual correlations among behaviors differed between treatments (Table 3). We found no evidence for incubation temperature driving differences among behavioral syndromes, with no among-individual correlations between any repeatable behavior (Table 3). This did not appear to be the result of developing in captivity, as we also did not detect relationships among behaviors in the fathers (Table 3).
Discussion
Our results show that developmental conditions affect the behavior of southern rainforest sunskinks, including mean behavior, repeatability, and within- and between-individual variation. Skinks incubated at a higher temperature were the most explorative, compared to those that developed at a lower temperature or in the wild. Effects on repeatability and behavioral variation were complex, with incubation temperature having opposite effects on different traits. Overall, differences in repeatability because of variation in developmental environment were primarily driven by changes in within-individual variation, which were higher in a greater number of traits for the high incubation treatment and lowest in the wild-caught group. We also found no evidence for a behavioral syndrome involving these behaviors in rainforest sunskinks, similar to previous studies on this species (Goulet et al. 2021), indicating that incubation temperature, as well as rearing in a captive compared to wild environment, does not affect between-individual correlations among behavioral traits. While our results further highlight the lasting effects of developmental conditions on an organism’s phenotype via behavioral responses, the fitness and ecological consequences of these effects remain unclear.
A higher incubation temperature resulted in southern rainforest sunskinks that were more explorative. Riskier behavioral types, such as exploration, can lead to a small but significant increase in survival in the wild, likely due to higher resource acquisition despite increased predation risk (Moiron et al. 2020). Our results, therefore, suggest a potential benefit to developing at a higher temperature, but contrast with previous studies. Three-toed skinks, Saiphos equalis, for example, incubated at a higher temperature spent less time exploring (Beltran et al. 2020), while pine snakes, Pituophis melanoleucus, incubated at both high and low temperatures were slower explorers than those incubated at a medium temperature (Burger 1998). Incubation temperature effects can differ in magnitude and direction in a trait- and species-dependent way (Noble et al. 2018; While et al. 2018), which may be due to differences between species in thermal adaptation or thermal preferences (Sinervo et al. 2010; Pincheira-Donoso et al. 2013; Beltran et al. 2020) and might lead to different impacts of climate change among species. It is, therefore, not unusual to see contrasting results between studies. The other behaviors we measured in our study did not, however, differ between incubation treatments. It may be our relatively small sample sizes playing a role, or, alternatively, some incubation temperature effects may not persist into adulthood, which has been found previously for critical thermal minimum in southern rainforest sunskinks (Llewelyn et al. 2018). However, other long-term studies have found incubation temperature effects lasting to 181–365 days post-hatching (Noble et al. 2018). It is more likely, therefore, that incubation temperature does not play a large part in determining mean behavioral phenotype of rainforest sunskinks, although with some exceptions, such as exploration.
We also found few differences in average behavior when comparing the captive-reared skinks to the wild-caught fathers. This was surprising, given the vast differences in developmental environments (Foster 2013), and previous studies demonstrating behavioral differences as a result of physical and social enrichment of rearing environments and as a result of predator cues in a wide range of taxa (e.g., reptiles: Riley et al. 2017; invertebrates: Liedtke et al. 2015; and amphibians: Urszan et al. 2015), both factors which would differ between captive and natural environments. However, garden skinks, L. guichenoti, reared with predator scent were found to become less responsive to the predator cues over time, such that after 1-year activity and microhabitat use did not differ from skinks reared with a control scent (Downes 2001). Similarly, juvenile but not adult southern water skinks, Eulamprus heatwolei, avoided predator odors (Head et al. 2002). Despite the age difference between captive- and wild-reared skinks in our study, all skinks were adults, which, if rainforest sunskinks also become less responsive behaviorally to predators with age, may explain the lack of difference in activity as well as sociability, as grouping behavior can provide antipredator benefits in skinks (e.g., delicate skinks: (Downes and Hoefer 2004). However, we did find lower exploratory behavior and a non-significant trend of lower boldness behavior in the wild-reared fathers. This trend may be due to riskier behavioral types more likely to persist in captivity but be predated upon in the wild (Smith and Blumstein 2008; Archard and Braithwaite 2010; Urszan et al. 2015), although does not explain why differences in exploration depended on the incubation temperature of the captive-reared skinks. It is possible that for southern rainforest sunskinks, the embryonic thermal environment, while not playing a large role in development of mean behavior, is still more important than differences present between captive and natural environments in contributing to adult phenotypes.
While we only found differences in mean exploratory behavior among incubation treatments, as well as captive—compared to wild-reared skinks, most behaviors differed in repeatability. This highlights the importance of developmental environments in shaping behavioral consistency, alongside studies investigating impacts of diet (Careau et al. 2014), predation (Urszan et al. 2015), and immune challenges (DiRienzo et al. 2015). For the repeatable behaviors, repeatability estimates fell broadly within the range of average repeatability, 0.37, found in a meta-analysis on a wide range of taxa (Bell et al. 2009). Interestingly, despite the above studies demonstrating that factors such as diet, predation, and immune challenges can affect repeatability, which would, among others, differ between natural and captive environments, behaviors where the wild-caught fathers demonstrated personality were the same behaviors where skinks from the low incubation treatment demonstrated personality. In contrast, there were more differences between the fathers and skinks incubated at the high temperature. While we cannot rule out some differences may be due to age variation between captive- and wild-reared skinks, this suggests a relatively large role of incubation temperature, among other factors varying during development, in determining personality in southern rainforest sunskinks.
Variation in personality between incubation treatments were largely driven by differences in within-individual variation, with differences in between-individual variation inconsistent, or non-existent, among behaviors. Skinks incubated at the high temperature had higher within-individual variation in four of the six behavioral measures, despite demonstrating personality in more behaviors, compared to skinks incubated at a low temperature. The only occasions where the higher incubation treatment had lower within-individual variation were for the behaviors where the high, but not low, incubation treatment demonstrated repeatability, i.e., latency to reach the goal compartment and sociability. Niemelä et al. (2019) demonstrated a similar trend in field crickets, Gryllus bimaculutas, where a higher developmental temperature increased within-individual variance in exploration behavior. However, crickets developing at this higher temperature also had greater between-individual variation (Niemelä et al. 2019). In comparison, in our study, southern rainforest sunskinks incubated at the higher temperature had increased between-individual variation for only one behavior, sociability, while those from the low incubation treatment had greater between-individual variation for two behavioral measures. This suggests that incubation temperature in skinks may have a greater effect on personality through affecting within-individual variation, i.e., the behavioral plasticity of individuals, potentially through different responses to trial number or habituation to the experimental assays, rather than affecting variation in behavioral types within populations. However, our sample sizes were relatively low, and we may therefore have not been able to fully capture more subtle impacts of incubation temperature on between-individual variation. Regardless, more plastic individuals are thought to be better suited to cope with environmental change (Visser 2008; Sih et al. 2011; Van Buskirk 2012), provided plasticity is adaptive rather than maladaptive or neutral. Therefore, as average temperatures, as well as incubation temperatures, rise with climate change, southern rainforest sunskinks may be better able to cope with short-term environment change as juveniles or adults.
Personality differences being driven by within-individual variation may be explained by early environments signaling that greater within-individual variation may be more beneficial in future environments. As such, developmental plasticity may affect short-term phenotypic changes expressed later in life, i.e., reversible plasticity. In mosquitofish, Gambusia holbrooki, for example, fish born in summer develop in, and are more likely to experience, warmer temperatures, and therefore have a lower ability to adjust metabolism in response to cooler temperatures experienced in adulthood, resulting in developmental plasticity affecting the ability to be reversibly plastic later in life (Seebacher et al. 2014). This reduces the potential of a phenotype-environment mismatch, and, if developmental environments reliably predict the need for reversible plasticity, may also reduce the trade-off with investment in other fitness-related traits, such as reproduction, as maintaining the ability to be plastic, such as more neural tissue or flexibility in physiology, can itself be costly (Snell-Rood 2013). While our study did not measure plastic responses of rainforest sunskinks in response to a change in the physical or social environment, the differences in within-individual variation observed among developmental treatments may have been the result of differences in individual variance in response to trial number or habituation to the behavioral assays. Alternatively, it may be more costly for individuals to maintain an ability to be more behaviorally variable or plastic. Higher costs of development, which can change, for example, with ectotherm developmental temperature (Marshall et al. 2020), or restricted resources during development, may therefore also affect investment in reversible plasticity. Increased diet quality in juvenile house crickets, for example, decreased repeatability through increased within-individual variation (Royauté and Dochtermann 2017), and, similarly, zebra finches, Taeniopygia guttata, reared without diet restrictions also had higher within-individual variance in activity (Careau et al. 2014). This may also explain the pattern of lower within-individual variation in the fathers in most behavioral measures compared to both incubation treatments, as conditions in captivity are generally more relaxed, for example, with consistent access to food and no predation risk, compared to what individuals experience in natural environments.
Despite repeatability in many behavioral traits, there was no evidence for a behavioral syndrome in southern rainforest sunskinks. This lack of between-individual correlations among behaviors is unsurprising, given Goulet et al. (2021) also did not find any correlations between activity, exploration, and sociability in southern rainforest sunskinks. However, our result contrasts with those from studies on the delicate skink, a closely related species, where activity, exploration, and, in some populations, boldness form a syndrome (Michelangeli et al. 2016b, 2019; Goulet et al. 2017). Many other studies have also found behavioral syndromes, suggesting they appear to be widespread among species (Wolf et al. 2007; Conrad et al. 2011; Wolf and Weissing 2012). There are many ways in which behaviors can correlate, and many ways in which these correlations might emerge (Réale et al. 2010). It is possible that these mechanisms are not present in southern rainforest skink habitats or that time in captivity has eroded or weakened relationships between behaviors (Wilson et al. 1994). Regardless, as neither incubation temperature group demonstrated significant correlations between behaviors, we can exclude developmental temperature as a factor driving the formation of behavioral syndromes, at least in southern rainforest sunskinks. However, incubation temperature could be important in the formation of behavioral syndromes in species that display such syndromes in wild populations, indicating an area requiring further research.
In summary, differences in incubation temperature affected behavioral variation and consistency of southern rainforest sunskinks, driven primarily through changes in within-individual variation. While skinks incubated at a higher temperature demonstrated personality in more behaviors, they also tended to have higher within-individual variation, specifically for behaviors where they were not the only treatment to demonstrate repeatability. We speculate that this may be due to lower costs of development, allowing them to develop a greater capacity for variation in behavior or that different incubation temperatures signal differences in future environments where higher within-individual variation may be more, or less, beneficial to individuals. Regardless, this may mean that skinks incubated at a higher temperature are more able to flexibly adjust to short-term environmental change. As environments are changing rapidly due to anthropogenic influences, such as habitat modification and climate change, this may be a mechanism for this group to better cope with these future environments. In contrast, mean behavior was not as strongly affected by developmental environment, with only differences between incubation treatments, as well as between captive- and wild-reared skinks, present in exploration. There was also no evidence that developmental temperatures affected between-individual correlations among behaviors, with no behavioral syndrome present in either incubation treatment. This did not appear to be the result of rearing in captivity, as there was also no behavioral syndrome present in the wild-caught fathers. Overall, our results demonstrate a large impact of rearing environment in shaping animal personality and behavioral variation, with incubation temperature a particularly significant factor in lizard behavioral development.
Data availability
The datasets generated and analyzed during the current study are available in the Bridges data repository, https://doi.org/10.26180/20057528.v1.
References
Archard GA, Braithwaite VA (2010) The importance of wild populations in studies of animal temperament. J Zool 281:149–160
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473
Bell A (2012) Randomized or fixed order for studies of behavioral syndromes? Behav Ecol 24:16–20
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Beltran I, Loiseleur R, Durand V, Whiting MJ (2020) Effects of early thermal environment on the behavior and learning of a lizard with bimodal reproduction. Behav Ecol Sociobiol 74:73
Blumstein DT, Daniel JC, Evans CS (2006) JWatcher 1.0, http://www.jwatcher.ucla.edu
Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192
Burger J (1998) Antipredator behaviour of hatchling snakes: effects of incubation temperature and simulated predators. Anim Behav 56:547–553
Burkner PC (2017) brms: An R package for Bayesian multilevel models using Stan. J Stat Soft 80:1–28
Careau V, Buttemer WA, Buchanan KL (2014) Early-developmental stress, repeatability, and canalization in a suite of physiological and behavioral traits in female zebra finches. Integr Comp Biol 54:539–554
Carere C, Drent PJ, Privitera L, Koolhaas JM, Groothuis TGG (2005) Personalities in great tits, Parus major: stability and consistency. Anim Behav 70:795–805
Chapple DG, Simmonds SM, Wong BBM (2011) Know when to run, know when to hide: can behavioral differences explain the divergent invasion success of two sympatric lizards? Ecol Evol 1:278–289
Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A (2011) Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J Fish Biol 78:395–435
Dingemanse NJ, Réale D (2005) Natural selection and animal personality. Behaviour 142:1159–1184
Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL (2012) Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J Anim Ecol 81:116–126
DiRienzo N, Niemela PT, Skog A, Vainikka A, Kortet R (2015) Juvenile pathogen exposure affects the presence of personality in adult field crickets. Front Ecol Evol 3:36
Downes S (2001) Trading heat and food for safety: Costs of predator avoidance in a lizard. Ecology 82:2870–2881
Downes S, Hoefer AM (2004) Antipredatory behaviour in lizards: interactions between group size and predation risk. Anim Behav 67:485–492
Downes S, Shine R (1998) Heat, safety or solitude? Using habitat selection experiments to identify a lizard’s priorities. Anim Behav 55:1387–1396
Downes SJ, Shine R (1999) Do incubation-induced changes in a lizard’s phenotype influence its vulnerability to predators? Oecologia 120:9–18
Dufty AM, Clobert J, Moller AP (2002) Hormones, developmental plasticity and adaptation. Trends Ecol Evol 17:190–196
Foster SA (2013) Evolution of behavioural phenotypes: influences of ancestry and expression. Anim Behav 85:1061–1075
Goulet CT, Thompson MB, Michelangeli M, Wong BBM, Chapple DG (2017) Thermal physiology: a new dimension of the pace-of-life syndrome. J Anim Ecol 86:1269–1280
Goulet CT, Hart W, Phillips BL, Llewelyn J, Wong BBM, Chapple DG (2021) No behavioral syndromes or sex-specific personality differences in the southern rainforest sunskink (Lampropholis similis). Ethology 127:102–108
Han CS, Dingemanse NJ (2015) Effect of diet on the structure of animal personality. Front Zool 12:S5
Head ML, Keogh JS, Doughty P (2002) Experimental evidence of an age-specific shift in chemical detection of predators in a lizard. J Chem Ecol 28:541–554
Johnson JC, Sih A (2007) Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolomedes triton. Anim Behav 74:1131–1138
Kar F, Nakagawa S, Noble DWA (2022) Impact of developmental temperatures on thermal plasticity and repeatability of metabolic rate. Evol Ecol 36:199–216
Lenth R (2020) emmeans: estimated marginal means, aka least-squares means, https://CRAN.R-project.org/package=emmeans)
Liedtke J, Redekop D, Schneider JM, Schuett W (2015) Early environmental conditions shape personality types in a jumping spider. Front Ecol Evol 3:134
Llewelyn J, Macdonald S, Hatcher A, Moritz C, Phillips BL (2017) Thermoregulatory behaviour explains countergradient variation in the upper thermal limit of a rainforest skink. Oikos 126:748–757
Llewelyn J, Macdonald SL, Moritz C, Martins F, Hatcher A, Phillips BL (2018) Adjusting to climate: acclimation, adaptation and developmental plasticity in physiological traits of a tropical rainforest lizard. Integr Zool 13:411–427
Marshall DJ, Pettersen AK, Bode M, White CR (2020) Developmental cost theory predicts thermal environment and vulnerability to global warming. Nat Ecol Evol 4:406–411
Michelangel M, Goulet CT, Kang HS, Wong BBM, Chapple DG (2018) Integrating thermal physiology within a syndrome: locomotion, personality and habitat selection in an ectotherm. Funct Ecol 32:970–981
Michelangeli M, Chapple DG, Wong BBM (2016a) Are behavioural syndromes sex specific? Personality in a widespread lizard species. Behav Ecol Sociobiol 70:1911–1919
Michelangeli M, Wong BBM, Chapple DG (2016b) It’s a trap: sampling bias due to animal personality is not always inevitable. Behav Ecol 27:62–67
Michelangeli M, Chapple DG, Goulet CT, Bertram MG, Wong BBM (2019) Behavioral syndromes vary among geographically distinct populations in a reptile. Behav Ecol 30:393–401
Moiron M, Laskowski KL, Niemelä PT (2020) Individual differences in behaviour explain variation in survival: a meta-analysis. Ecol Lett 23:399–408
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Niemelä PT, Niehoff PP, Gasparini C, Dingemanse NJ, Tuni C (2019) Crickets become behaviourally more stable when raised under higher temperatures. Behav Ecol Sociobiol 73:81
Noble DWA, Stenhouse V, Schwanz LE (2018) Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol Rev 93:72–97
Norton WHJ, Stumpenhorst K, Faus-Kessler T, Folchert A, Rohner N, Harris MP, Callebert J, Bally-Cuif L (2011) Modulation of fgfr1a signaling in zebrafish reveals a genetic basis for the aggression-boldness syndrome. J Neurosci 31:13796–13807
Pincheira-Donoso D, Tregenza T, Witt MJ, Hodgson DJ (2013) The evolution of viviparity opens opportunities for lizard radiation but drives it into a climatic cul-de-sac. Glob Ecol Biogeogr 22:857–867
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063
Rhen T, Sakata IT, Crews D (2005) Effects of gonadal sex and incubation temperature on the ontogeny of gonadal steroid concentrations and secondary sex structures in leopard geckos, Eublepharis macularius. Gen Comp Endocrinol 142:289–296
Riley JL, Noble DWA, Byrne RW, Whiting MJ (2017) Early social environment influences the behaviour of a family-living lizard. R Soc Open Sci 4:161082
Royauté R, Dochtermann NA (2017) When the mean no longer matters: developmental diet affects behavioral variation but not population averages in the house cricket (Acheta domesticus). Behav Ecol 28:337–345
Royauté R, Buddle CM, Vincent C (2015) Under the influence: sublethal exposure to an insecticide affects personality expression in a jumping spider. Funct Ecol 29:962–970
Seebacher F, Beaman J, Little AG (2014) Regulation of thermal acclimation varies between generations of the short-lived mosquitofish that developed in different environmental conditions. Funct Ecol 28:137–148
Shine R (2003) Effects of pregnancy on locomotor performance: an experimental study on lizards. Oecologia 136:450–456
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Sinervo B, Mendez-de-la-Cruz F, Miles DB et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455
Snell-Rood EC (2013) An overview of the evolutionary causes and consequences of behavioural plasticity. Anim Behav 85:1004–1011
Sokolowski MB (2001) Drosophila: genetics meets behaviour. Nat Rev Genet 2:879–890
Therneau TM (2020) coxme: mixed effects cox models. R package version 2.2–16, https://CRAN.R-project.org/package=coxme
Urszan TJ, Garamszegi LZ, Nagy G, Hettyey A, Torok J, Herczeg G (2015) No personality without experience? A test on Rana dalmatina tadpoles. Ecol Evol 5:S847–S856
Van Buskirk J (2012) Behavioural plasticity and environmental change. In: Wong BBM, Candolin U (eds) Behavioural responses to a changing world: Mechanisms and consequences. Oxford University Press, Oxford, pp 145–158
Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc R Soc Lond B 275:649–659
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York
While GM, Noble DWA, Uller T, Warner DA, Riley JL, Du WG, Schwanz LE (2018) Patterns of developmental plasticity in response to incubation temperature in reptiles. J Exp Zool A 329:162–176
Wilson DS, Clark AB, Coleman K, Dearstyne T (1994) Shyness and boldness in humans and other animals. Trends Ecol Evol 9:442–446
Wilson S, Swan G (2021) A complete guide to the reptiles of Australia. Reed New Holland, Chatswood, NSW
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life-history trade-offs favour the evolution of animal personalities. Nature 447:581–584
Acknowledgements
We thank C. Goulet, G. Matthews and other lab members for their assistance with captive lizard husbandry, and R. de Jong for help with experimental equipment. We also thank two anonymous reviewers for their constructive feedback during the revision process.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by an Australian Research Council Discovery Project Grant (to DGC and BBMW; DP170100684).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Monash University Animal Ethics Committee (BSCI/2013/19 and BSCI/2016/02).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by S. Joy Downes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David G. Chapple and Bob B. M. Wong are Joint Senior Authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Jong, M., Phillips, B.L., Llewelyn, J. et al. Effects of developmental environment on animal personality in a tropical skink. Behav Ecol Sociobiol 76, 137 (2022). https://doi.org/10.1007/s00265-022-03240-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03240-3