Abstract
How and when deimatic behaviours are performed can change during encounters between predators and prey. Some predators attack repeatedly, investigating and manipulating prey, and in response, an individual’s deimatic behaviour may intensify or may diminish in favour of escaping. The presence of a resource can further force a trade-off between displaying and escaping. Here, we examined the intensity of the katydid’s deimatic behaviour, a visual display, the propensity of their escape response under repeated simulated attacks, and how these responses change in the presence of foraging resources. We found that display intensity increased with repeated simulated attacks and that females displayed at a greater intensity than males. The presence of their preferred food plant had no significant effect on display intensity, but reduced escape probability in both sexes. Some katydids were predictable in their display intensity and at the population level we found that strong display intensity is moderately repeatable. Overall, our results suggest that 1) display intensity increases with repeated attacks and might indicate a cost in performing at maximum intensity upon first attack, 2) deploying a deimatic display while feeding can reduce the need to flee a rich foraging patch and 3) some individuals are consistent in their display intensities. Future experiments that aim to determine causal mechanisms such as limitations to perception of predators, sensitisation to stimuli and physiological constraints to display intensity will provide necessary insight into how deimatic displays function.
Significance statement
Though often regarded as success or failure, interactions between predators and prey during the attack phase of a predation event are complex, especially when predators make repeated investigative attacks in quick succession. Our study shows that in mountain katydids, intensity of deimatic behaviour increases with repeated attacks, perhaps indicating that prey sensitise or that maximal displays during initial attacks carry high costs such as conspicuousness. The intensity of the display does not change with the introduction of a valuable food resource, but the probability of fleeing decreased, suggesting that displaying may reduce the opportunity costs of leaving a patch. We also show that individuals vary in the repeatability of their display, suggesting that deimatic display may be highly adaptable, nuanced and targeted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strong selective pressure imposed on prey by their predators has resulted in the evolution of a diverse array of adaptations to interrupt the predation sequence (Edmunds 1974; Endler 1991; Ruxton et al. 2019). When discovered, prey may attempt to flee by dropping or running away to create distance from the predator (Humphreys and Ruxton 2018; Konishi et al. 2020), freeze in place (Eilam 2005; Bedore et al. 2015; York and Bartol 2016) or deploy defences such as deimatic displays and chemical sprays (Eisner and Aneshansley 1999; Endler and Mappes 2004; Mattila et al. 2020). The decision to either defend or escape is made by weighing the costs associated with each behaviour, such as the risk of a predator overcoming defences and the energetics of escaping (Ydenberg and Dill 1986). When the cost of abandoning a resource outweighs the perceived risk of succumbing to predation, individuals may choose to stay and defend. For example, redshanks (Tringa totanus) must avoid predation from both sparrowhawks (Accipiter nisus) and peregrines (Falco peregrinus), when foraging highly profitable, high-risk saltmarsh areas. To increase their survival while not giving up valuable resources, individuals employ antipredator behaviours such as vigilance, spacing and careful positioning within the flock, rather than fleeing (Sansom et al. 2009; Turney and Godin 2014).
Deimatic behaviours are performed when prey perceive a threat and exploit the predator’s threat response causing it to slow or stop its attack (Bura et al. 2011; Kang et al. 2016; Umbers et al. 2017, 2019) (Drinkwater et al. 2022). Deimatic behaviours are highly variable across animals, but this variation can be pronounced even among closely related species (Schlenoff 1985; Sargent 1990; Vidal-García et al. 2020; Kim et al. 2020). For example, in Catocala moths, hidden hindwing colouration varies among sympatric species, and predators trained to expect one colour can be deterred when they are presented with a different colour (Schlenoff 1985). Moreover, displays can vary substantially within a species, because individuals make decisions not only about whether to display or not, but also on how intensely to display: this can be achieved by modulating how many components of their display are deployed, for how long and how many times. For example, different stimuli can elicit displays of different intensities in mountain katydids and praying mantises, which display more intensely when they encounter a tactile stimulus in comparison with visual or auditory ones (Umbers and Mappes 2015; O’Hanlon et al. 2018).
Predation attempts in the wild are difficult to observe, and it is often assumed that predators ambush and attack prey very fast, killing before the prey realise the predator is present (Schmitz et al. 1997; Nordberg and Schwarzkopf 2019). However, close observation reveals that many predators approach prey with caution, make probing investigations before delivering the fatal blow (Endler 1978; Bateman et al. 2014). In this type of interaction, prey may experience repeated attacks and perform their display several times in a row. The Northern Bluetongue skink (Tiliqua scincoides intermedia), for example, displays a conspicuously blue-coloured tongue in response to predation. Their deimatic display increases in intensity, from tongue flicks to full tongue displays, when repeatedly and rapidly attacked by an avian predator (Badiane et al. 2018).
Individuals do not necessarily respond consistently to a given scenario, and even the same stimulus can elicit different responses in the same individual (Rosas et al. 2013). Repeatability in individuals has been shown across many taxa (Kok et al. 2019, Montiglio et al. 2012; Herde and Eccard 2013, Fuiman and Cowan 2003 including in other insect species (Kent and Rankin 2001; Missoweit et al. 2007). Repeatability in behavioural responses can affect our understanding of the dynamics and impacts of predator–prey models and can measure the variation of an individual behaviour over time (McCabe 2017). For example, in individual Bighorn sheep (Ovis canadensis) temperament was found to be repeatable, with the same individual consistently showing bold or docile traits. Individuals that were consistently bold were less susceptible to predation over time in comparison with individuals that were not consistent in their temperament (Réale and Festa-Bianchet 2003). Understanding repeatability can help explain individual behavioural decisions to escalate or deescalate an antipredator encounter (Briffa and Greenaway 2011). Cane toads (Rhinella marina) escalate their antipredator display (willingness to flee) fleeing more rapidly, following repeated attacks from predators (Hudson et al. 2017). Studies to date have typically assumed, when measuring deimatic displays, that each response was independent of the previous (Umbers and Mappes 2015; De Bona et al. 2020), but understanding whether prey sensitise or desensitise, and escalate or deescalate, in response to attacks made in quick succession remains an unexplored question.
In this study, we examined the effect of repeated simulated predation attacks on the deimatic display and escape response of the mountain katydid (Acripeza reticulata). We hypothesised that the display intensity of individual katydids would change with simulated quick-succession predation attempts. We predicted that their response would escalate or deescalate depending on whether katydids sensitise or desensitise to repeated attacks. Our rationale for escalation was that katydids may be reluctant to perform a full display upon first perception of an attack if their display is costly in terms of conspicuousness to eavesdroppers or if the chances of displaying in response to a stimulus that is in fact non-threatening are high. Our rationale for de-escalation was that katydids may display maximally immediately on attack and, upon experiencing subsequent non-lethal attacks, disregard the stimulus as a serious threat. We further hypothesised that the presence of a foraging resource would change the display and escape behaviour of katydids. We predicted that in the presence of a resource, katydids would choose to escape less often than in the absence of it and that display intensity would increase to increase defences and compensate for the choice to stay. Finally, to investigate behavioural consistency in display responses, we asked whether individual katydid display intensities are repeatable across independent trials to investigate individual flexibility as a source of display intensity variation.
Methods
Study species
Mountain katydids (Acripeza reticulata Guérin-Méneville 1832) are a large diurnal orthopteran (Rentz 1996), especially common in montane and sub-alpine habitats in eastern Australia. When at rest, mountain katydids are camouflaged due to their mottled dark brown forewings. When attacked, katydids raise their wings rapidly to reveal striking red, blue and black bands on the dorsal surface of the abdomen (Fig. 1).
The abdominal colouration is sometimes displayed together with an antenna waving display and reveal of an orange intersegmental membrane between the head and the pronotum (Umbers and Mappes 2015). When displaying, the katydid discharges an alkaloid-rich secretion from its abdomen and regurgitates bitter crop fluids as a form of chemical defence (Cable and Nocke 1975; Baker 2019; Umbers et al. 2019). The mountain katydid is sexually dimorphic, with the males being small (ca. 1 g) and flight-winged, while the females are larger (ca. 3 g) and flightless (De Bona et al. 2020).
Katydid husbandry
We collected 76 mountain katydids, 42 females and 34 males, from Kosciuszko National Park in April of 2015 under New South Wales National Parks and Wildlife Service Scientific Licence Number SL101474. Following De Bona et al. (2020), the katydids were housed in a group mesh enclosure of 1.5 × 0.4 × 0.4 m outdoors at our accommodation in Thredbo Village, Kosciuszko National Park. All katydids were held for a total of eight days: five days prior to testing, which was then conducted over a period of two days. Katydids were provided with water ad libitum in two ways—on cotton wool in their enclosure and by emulating morning dew by spraying water onto the food plants Senecio gunnii and Senecio pinnatifolius in their enclosure (De Bona et al. 2020). Each katydid collected was individually identified by attaching a unique bee tag to the tegmina (Pender’s Bee Supplies, Cardiff, NSW, Australia) within the first 48 h of their capture, kept for the period of the experiments and sacrificed and preserved in 70% ethanol at the end of the experiment.
Behavioural assays
To explore whether katydid deimatic behaviour changes when exposed to repeated attacks, we simulated repeated predation attempts within an arena. The arena consisted of a 1-cm gridded cardboard sheet placed on a hard, flat surface. We marked the centre of the arena using three concentric circles at a radii 25 mm, 150 mm and 300 mm to provide landmarks for retuning the katydid to the middle of the arena and thresholds over which katydids were considered to have escaped. Prior to each trial, katydids were acclimated for a period of at least 30 min at 23–25 °C in their mesh housing enclosure. Once they had acclimated, they were selected haphazardly and placed in the middle of the arena and, pinched nine further times, once every 10 s, to stimulate repeated attacks using an interval timer. The initial removal from the enclosure and placement in the arena was counted as the first attack, and the second attack was made after 10 s and continued for further eight attacks for a total of 10 repeated attacks. After each simulated attack, the katydid was placed back in the centre of the arena and the display intensity recorded as the number of red stripes displayed (Fig. 1). To stimulate an attack, pressure was applied in a pinching motion to the pronotum using the index finger and thumb. As observed in the field, this pinching method was meant to stimulate an avian predator’s attacking behaviour, to elicit the natural defensive response of the katydid (Umbers and Mappes 2015; Umbers et al. 2019). The same researcher (K.U.) handled the katydids each trial to ensure approximately the same force was used. In order to standardise the force of the pinch used in these trials, we practiced (Blumstein et al. 2018) on other insects and our preliminary attempts to elicit responses from a different group of katydids that were not used in this experiment.
In a second round of simulated attacks, we added a food resource at the centre of the arena to determine whether katydid responses change when they have a higher motivation to remain in place. After the initial round of experiments, the same katydids were starved for 24 h before the second round of trials to increase motivation and the value of the food resource. We applied the same procedure in the first round of experiment, to this experiment; however, we placed a sprig of the katydid’s food plant S. linariifolius in the centre starting point of the arena.
Scoring display and escape behaviours
To measure display intensity, we first scored the intensity of the display as an ordinal categorical score reflecting the number of abdominal red bands visible when the katydid displayed (Fig. 1), which ranged from a minimum of zero visible bands to a maximum of three (Umbers and Mappes 2015).
To estimate katydid’s propensity for escape, we recorded if the katydid moved from the centre of the arena to cross the inner (150 mm) ring. Katydids were considered to have crossed this ring when the first foot passed over the line. Each trial was filmed using a Sony Camcorder (HXR-NX30P NXCAM) fixed above the arena with a top-down view and, as a back-up, using two GoPros (HD Hero4 Action Video Camera, GPCHDHY-401; GoPro Inc., www.gopro.com) set on two opposing corners of the arena. In order to analyse the escape response of the katydids, we measured whether or not katydids exited the rings drawn at 150 mm on the floor of the arena during the 10-s interval between attacks.
Statistical analysis and model fitting
Throughout the analyses, we maintained the same model simplification approach. We started from a model containing all main effects of interest and all their two-way interactions and three-way interaction (when three effects were present). We proceeded by removing interactions hierarchically (three-way first, then two-way) if they were not significant, in order of significance (higher to lower p-value). We never removed main effects. When an interaction was dropped, we compared the models through a likelihood ratio test (LRT) to ensure removing the interaction did not reduce the model fitness. If the presence of the interaction did not significantly increase model fit, we retained the model without interaction, following parsimony (Supplementary information: Tables S1–S7). All analyses were conducted in RStudio (R Core Team 2020, v. 1.13). The data were organised using packages reshape2 (Wickham 2020, v. 1.4.4), stringr (Wickham 2019, v. 1.4.0) and dplyr (Wickham et al. 2020, v. 1.0.2) and analysed using packages lme4 (Bates et al. 2015, v. 1.1–21) and ordinal (Christensen 2018).
Display intensity
To analyse the intensity of the display response at each repeated attack, we fit a cumulative link mixed model (CLMM) with a logistic link function. The display intensity score, taking values of 0, 1, 2 or 3, and representing the number of abdominal stripes shown, was adopted as our ordinal response variable. We included individual identity as a random effect to account for our repeated measures design. We included sex as an explanatory variable in the model to examine sex-specific differences and attack number (referring to the order of the attack along the sequence of repeated attacks, ranging from 1 to 10) as a continuous variable, to determine the effect of repeated attacks. We included the presence of food resource (plant: 1 = present, 0 = absent) as an additional explanatory variable to study how the presence of a resource affects display intensity. We first fit the model including all two- and three-way interactions among the three explanatory variables and then simplified it as described above (Supplementary information: Tables S1 to S4). In addition, to account for the potential presence of a ceiling effect occurring after a set number of subsequent attacks, we repeated the analyses only including the first 7, 5, and 3 attacks.
Escape propensity
In order to determine whether the propensity to escape during the predation simulation was influenced by repeated attacks, display intensity, sex and the presence of a resource, we modelled escape propensity using a GLMM with a binomial error distribution and logit link function. We included escape as a binary response variable (where 1 = escaped, 0 = stayed) and attack number (1 to 10) as a continuous variable, sex and plant presence as explanatory variables. We included individual identity as a random effect. We first fit all two- and three-way interactions among these three explanatory variables and proceeded to simplify the model using the same approach described above (Supplementary information: Tables S5 to S7).
Are individual katydid display intensities repeatable?
While conducting experiments on the responses of katydids to multiple attacks, we observed that some individual katydids gave consistent displays of similar intensity. In particular, we noticed individual katydids displaying at maximum intensity tended to do so across trials. To determine whether strong display intensity is repeatable at the population level, we took display responses to the first stimulated attack for five consecutive days. Three days originated from a previous experiment the experiment presented in De Bona et al 2020, to which we added the first response from simulated attacks conducted on the two days of the experiments described here (without and with plants). We transformed the ordinal behavioural response to a binomial response, where 1 represents a maximum intensity response (a value of 3 in the previous categorisation) and 0 represents any other response. We did so to overcome statistical hurdles deriving from the challenge of calculating residual variance in an ordinal response model (Schmidt et al. 2013). Following recommendations (Nakagawa and Schielzeth 2010) on how to calculate repeatability in behaviour, we fit a GLMM with a logit link function, we modelled a repeatability estimation to determine whether katydid maximum display intensity was repeatable within individuals, adding individual identity as a random effect using the rptR package [v0.9.22; (Stoffel et al. 2017)].
Results
What is the effect of repeated simulated attacks on display intensity and is this affected by the presence of a resource?
Display intensity varied with sex and with consecutive attacks (Fig. 2). Overall, females displayed at higher intensity than males across all 10 attacks (Table 1), with half or more females showing three stripes in almost every trial (with the only exception of trial one when plant food was present). We found that, for both sexes, display intensity increased with consecutive attacks. This was reflected in the significant effect of attack number, which had a moderate relative effect size (Table 1). The presence of the food resource did not significantly affect display intensity, but the effect differed between the two sexes (Fig. 2, Table 1). The analyses including only the first 7, 5, or 3 attacks yielded consistent results, highlighting that the escalation of display intensity occurred rapidly along the sequence of attacks (Supplementary Information: Tables S8 to S10).
Observed display intensity of female (a) and male (b) katydids across the 10 attack replicates in an arena without plants (grey) and in an arena with plants (green), repeated across three consecutive trials. Predicted probability of each display score intensity for females (c) and males (d) according to the model. In all panels, plant presence is in green and absence in grey
What is the effect of repeated simulated attacks on the propensity of escape and is it altered by the presence of a resource?
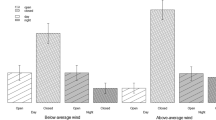
In the absence of any resources in the arena, neither attack nor sex influenced the propensity of katydids to escape (Fig. 3, Table 2). Females started at a 77% estimated probability of escape in the first attack, which that increased to 88% by the tenth and final attack (Fig. 3). Males initially had a 52% estimated probability of escaping which increased to 78% by the tenth attack. However, escape propensity varied with the presence of a food resource (Fig. 3). Initially, the probability of escape when a plant food resource was present was significantly lower than without the plant and remained lower through the repeated attacks (Table 2). For females, the estimated probability of escape started at 11% at the first attack and increased to 55% by the tenth attack replicate (Fig. 3). In the trials with a plant present, males had an even lower estimated probability of escape than females, 5%, which increased by the tenth attack to 36%.
Are individual katydid displays repeatable?
Using a GLMM model, we determined the repeatability estimate of maximum display intensity in 75 individual katydids (Fig. 4). We found significant moderate repeatability in the original-scale approximation (repeatability (r [± 95% CI]) = 0.493 [0.237 – 0.739], standard error (SE) = 0.131, P = < 0.01). The link-scale approximation estimate likewise showed significant moderate repeatability (r [± 95% CI] = 0.433 [0.216 – 0.575], SE = 0.092 P = < 0.01).
Discussion
Our results show that mountain katydids increase the intensity of their display in response to consecutive simulated attacks. Display intensity was greater for females than males but increased at a similar rate in both sexes over the attacks. In terms of escape behaviour, females were more likely to escape when attacked than males, but for both sexes the probability of escape increased with subsequent attacks. The presence of a food resource did not change katydid display intensity but markedly decreased the probability of both males and females escaping after the first attack. However, even with the food resource present, the probability of escape increased over the attacks. This suggests that display intensity increases as perceived risk from a threat increases, while escape behaviour is driven more by the richness of a patch. In terms of consistency in katydid responses, we found greater repeatability in display intensity than expected by chance and overall low to moderate levels of repeatability, suggesting that while some katydids are more likely to perform displays at a consistent intensity, most are more variable in their response.
Mountain katydid flight or fight response to repeated attacks
In response to repeated stimulated predation, we found katydids to increase their display intensity. The mechanism for this increase might be sensitisation, a non-associative learning process, in which repeated contact to a stimulus as time progresses, causes an organism to increase their reaction to a stimulus (Wells 1968; Watkins et al. 2010). Attacks by predators can trigger the sensitisation or desensitisation of defensive responses in prey species and result in an increase or decrease, respectively, of these responses (Walters 1994; Crook et al. 2014). For example, predator attacks on sea hares (Aplysia californica) cause sensitisation, resulting in an increase in the reflex withdrawal intensity of the tail-mantle and head (Watkins et al. 2010). In terms of ecological drivers, we hypothesise that the conspicuousness costs may be too high for mountain katydids to display maximally when they are first attacked. Future studies could test whether they pay a conspicuousness cost for displaying too intensely too early in the predation sequence. Circumstantial evidence to support this idea comes from observations of the predatory behaviour of the mountain katydid’s known predators, Australian magpies (Gymnorhina tibicen). Magpies typically perform several investigative non-lethal pecks initially, which the katydids might be able to withstand due to the toughness of their tegmina (Umbers et al. 2019).
Display intensity was greater in females than in males throughout the repeated predation trials, consistent with previous work on mountain katydids and possibly reflective of their different morphology (Umbers and Mappes 2015; De Bona et al. 2020). As the wings of adult male katydids are bigger than those of females, they need to be lifted at a wider angle to reveal the defensive signals, perhaps increasing the cost of performing the display. Males also have much smaller abdomens and therefore a smaller defence signal and could gain less from a deimatic strategy in comparison with females (Umbers and Mappes 2015). There are many noted examples of differences in antipredator defences between sexes in Arthropods that stem from sexual dimorphism (Conroy and Gray 2015; Tanis et al. 2018; Segovia et al. 2019). In the harvestmen Mischonyx cuspidatus males, for example, employ different antipredator strategies to those of females, using a pair of sharp apophyses, while female, who lack them, use thanatosis (Segovia et al. 2019). There are also significant differences in antipredator behaviours in stalk-eyed flies (Teleopsis dalmanni) where males’ exaggerated large eye spans allow for an aggressive physical ‘jabbing’ behaviour to deter predators (Worthington and Swallow 2010). Similar sex differences in deimatic display intensity are found in praying mantises in which females have more elaborate and intense displays (O’Hanlon et al. 2018). Such differences in display behaviour between the sexes could be an indirect effect of their morphology, which defines escape capacity; female katydids and mantises have limited flight abilities, while the males are smaller and can fly surprisingly well (Umbers and Mappes 2015; De Bona et al. 2020).
Mountain katydid flight or fight response to repeated attacks in the presence of a food resource
We asked whether the katydids display at different intensities in the presence of a food source. Overall, the presence of the plants did not change the katydids’ display intensity (Fig. 2, Table 1).
While display intensity did not change in the presence of the food resource, escape propensity did, with katydids less likely to escape when a food resource was available (Fig. 3, Table 2). Reluctance to escape when foraging is also seen in other animals such as mountain lizards (Liolaemus monticola), Iberian rock lizards (Iberolacerta monticola) and Balearic lizards (Podarcis lilfordi) all of which allow the closer approach of a predator before escaping in the presence of a food source compared with trials without food, suggesting that there is a trade-off between foraging and predation risk (Cooper 2003; Cooper and Peréz-Mellado 2004; Cooper et al. 2006). The lizards’ responses to repeated attacks are also similar to our results in the mountain katydid, as repeated attacks caused lost opportunity for foraging due to an increased flight initiation distance and longer time spent hiding from predators in refuges (López and Martín 2001; Martín et al. 2009). The mechanism by which repeated attacks are thought to increase antipredator vigilance is by reinforcing the lizard’s perception of risk (Cooper 1998; Martín and López 2004). Aspic vipers (Vipera aspis) also escape and seek refuge in response to predator encounters, but when given a choice between hiding and basking (thermal resource), the likelihood of fleeing increases (Lorioux et al. 2013). Katydids appear to try and solve the trade-off between foraging and fleeing by performing their display at high intensity. In our study, the attacks were simulated by us and therefore the katydid’s display could not be successful; however, field-based data on the success of displays deterring predators and allowing katydids to continue foraging would be of great interest.
The repeatability of individual katydids in display intensity
Our analysis shows that mountain katydids have moderate repeatability of their maximum display intensity (Harper 1994; Cauchoix et al. 2018). Some katydids were more likely to always perform a lower intensity display, while others are more likely to perform a high intensity display, and there was considerable variation within individuals (Figs. 2 and 4). While some individual katydid displays were extremely consistent, other katydids varied in the intensity of their display over the repeated trials, suggesting that their response to simulated attack is not fixed but flexible over the five-day time frame (Umbers et al. 2019; De Bona et al. 2020). These moderate repeatability results suggest the insects’ responses are flexible and may decay temporally or be based on environmental variation and be highly context dependant. Over what time frames, and exactly what environmental conditions influence the expression of display behaviour is unclear, but could include different kinds of predators, body condition or abiotic factors like ambient temperature.
Finally, it is possible that repeatability of katydid display was influenced by the artificial conditions under which our experiment took place. Studies that measure repeatability in the field seem to show higher repeatability in comparison with those done in a controlled setting (Bell et al. 2009). For example, in field crickets (Gryllus campestris) behavioural traits measuring exploration had a lower repeatability in the wild than in a laboratory setting (Fisher et al. 2015). There are also examples where the setting of the experiment does not affect repeatability. For example, in beadlet anemones (Actinia equine) repeatability estimates in the laboratory and in the wild are very similar (Osborn and Briffa 2017). The katydids used in our study were captured from the wild and held captive for five days before being tested, a process that may have influenced display repeatability. We suggest future studies attempt to assess repeatability in the wild to ground-truth our results.
Our results provide evidence that the intensity of a deimatic display increases under repeated sequential predation events. While access to a food recourse does not affect display intensity, it does decrease the katydid’s propensity to flee after repeated predation, highlighting the context dependence of anti-predatory responses. The moderate within-individual repeatability of display intensity further hints at the fact that display intensity may be dependent on external environmental context rather than internal drivers. The next steps to further understand the antipredator behaviour of A. reticulata would be to explore the efficacy of the two defence strategies (escape and deimatic displays) in deterring real predators. Since external factors appear to play a role in determining the escape response, predator identity could also affect the mode of defence. Testing the defensive reaction to multiple predators could shed light on this question.
Data availability
Supplementary file.
Code availability
Supplementary file.
References
Badiane A, Carazo P, Price-Rees SJ et al (2018) Why blue tongue? A potential UV-based deimatic display in a lizard. Behav Ecol Sociobiol 72:104. https://doi.org/10.1007/s00265-018-2512-8
Baker B (2019) Antipredator chemical defence in the mountain katydid (Acripeza reticulata). Masters Thesis, Western Sydney University
Bateman PW, Fleming PA, Rolek B (2014) Bite me: Blue tails as a “risky-decoy”defense tactic for lizards. Curr Zool 60:333–337
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bedore CN, Kajiura SM, Johnsen S (2015) Freezing behaviour facilitates bioelectric crypsis in cuttlefish faced with predation risk. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2015.1886
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77(4):771–783
Blumstein DT, Diaz A, Yin L (2018) Marmots do not consistently use their left eye to respond to an approaching threat but those that did fled sooner. Curr Zool 64:727–731. https://doi.org/10.1093/cz/zoy003
Briffa M, Greenaway J (2011) High In Situ Repeatability of Behaviour Indicates Animal Personality in the Beadlet Anemone Actinia equina (Cnidaria). PLoS One 6:e21963. https://doi.org/10.1371/journal.pone.0021963
Bura VL, Rohwer VG, Martin PR, Yack JE (2011) Whistling in caterpillars (Amorpha juglandis, Bombycoidea): sound-producing mechanism and function. J Exp Biol 214:30–37. https://doi.org/10.1242/jeb.046805
Cable J, Nocke H (1975) Isolation of s-Butyl β-D-glucopyranoside from Acripeza reticulata. Aust J Chem 28:2737–2739. https://doi.org/10.1071/ch9752737
Cauchoix M, Chow PKY, van Horik JO, et al (2018) The repeatability of cognitive performance: a meta-analysis. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2017.0281
Christensen R (2018) ordinal - Regression Models for Ordinal Data. Version R package version 2018.4–19URL http://www.cran.r-project.org/package=ordinal/
Conroy LP, Gray DA (2015) Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects 6:85–99. https://doi.org/10.3390/insects6010085
Cooper WE Jr (1998) Risk factors and emergence from refuge in the lizard Eumeces laticeps. Behaviour 135:1065–1076. https://doi.org/10.1163/156853998792913465
Cooper WE (2003) Effect of Risk on Aspects of Escape Behavior by a Lizard, Holbrookia propinqua, in Relation to Optimal Escape Theory. Ethology 109:617–626. https://doi.org/10.1046/j.1439-0310.2003.00912.x
Cooper WE, Peréz-Mellado V (2004) Tradeoffs between escape behavior and foraging opportunity by the balearic lizard (podarcis lilfordi). Herpetologica 60:321–324. https://doi.org/10.1655/04-20
Cooper WE, Pérez-Mellado V, Hawlena D (2006) Magnitude of food reward affects escape behavior and acceptable risk in Balearic lizards, Podarcis lilfordi. Behav Ecol 17:554–559. https://doi.org/10.1093/beheco/arj066
Crook RJ, Dickson K, Hanlon RT, Walters ET (2014) Nociceptive Sensitization Reduces Predation Risk. Curr Biol 24:1121–1125. https://doi.org/10.1016/j.cub.2014.03.043
De Bona S, White TE, Umbers KDL (2020) Fight or flight trade-offs and the defensive behaviour of the mountain katydid, Acripeza reticulata. Anim Behav 159:81–87. https://doi.org/10.1016/j.anbehav.2019.11.012
Drinkwater E, Allen WL, Endler JA, Hanlon RT, Holmes GG, Homziak NT, Kang C, Leavell BC, Lehtonen J, Loeffler-Henry K, Ratcliffe JM, Rowe C, Ruxton GD, Sherratt TN, Skelhorn J, Skojec C, Smart HR, White TE, Yack JE, Young CM, Umbers KDL (2022) A synthesis of deimatic behaviour. Biological Reviews. https://doi.org/10.1111/brv.12891
Edmunds M (1974) Defence in Animals: A Survey of anti-predator defences. Longman, London
Eilam D (2005) Die hard: a blend of freezing and fleeing as a dynamic defense–implications for the control of defensive behavior. Neurosci Biobehav Rev 29:1181–1191. https://doi.org/10.1016/j.neubiorev.2005.03.027
Eisner T, Aneshansley DJ (1999) Spray aiming in the bombardier beetle: Photographic evidence. Proc Natl Acad Sci 96:9705–9709. https://doi.org/10.1073/pnas.96.17.9705
Endler JA (1991) Interactions between predators and prey. In: Behavioural ecology: an evolutionary approach. Wiley-Blackwell Publishing, Hoboken, pp 169–196
Endler JA (1978) A Predator’s View of Animal Color Patterns. In: Hecht MK, Steere WC, Wallace B (eds) Evolutionary Biology. Springer US, Boston, pp 319–364
Endler JA, Mappes J (2004) Predator Mixes and the Conspicuousness of Aposematic Signals. Am Nat 163:532–547. https://doi.org/10.1086/382662
Fisher DN, David M, Tregenza T, Rodríguez-Muñoz R (2015) Dynamics of among-individual behavioral variation over adult lifespan in a wild insect. Behav Ecol 26:975–985. https://doi.org/10.1093/beheco/arv048
Fuiman L, Cowan J (2003) Behavior and recruitment success in fish larvae: Repeatability and covariation of survival skills. Ecology 84:53–67. https://doi.org/10.1890/0012-9658(2003)084[0053:BARSIF]2.0.CO;2
Harper DGC (1994) Some comments on the repeatability of measurements. Ringing Migr 15:84–90. https://doi.org/10.1080/03078698.1994.9674078
Herde A, Eccard JA (2013) Consistency in boldness, activity and exploration at different stages of life. BMC Ecol 13:49. https://doi.org/10.1186/1472-6785-13-49
Hudson CM, Brown GP, Shine R (2017) Evolutionary shifts in anti-predator responses of invasive cane toads (Rhinella marina). Behav Ecol Sociobiol 71:1–9
Humphreys RK, Ruxton GD (2018) A review of thanatosis (death feigning) as an anti-predator behaviour. Behav Ecol Sociobiol 72:22. https://doi.org/10.1007/s00265-017-2436-8
Kang C, Cho H-J, Lee S-I, Jablonski PG (2016) Post-attack aposematic display in prey facilitates predator avoidance learning. Front Ecol Evol 4:1–9. https://doi.org/10.3389/fevo.2016.00035
Kent JW, Rankin MA (2001) Heritability and physiological correlates of migratory tendency in the grasshopper Melanoplus sanguinipes. Physiol Entomol 26:371–380. https://doi.org/10.1046/j.0307-6962.2001.00257.x
Kim Y, Hwang Y, Bae S et al (2020) Prey with hidden colour defences benefit from their similarity to aposematic signals. Proc R Soc B Biol Sci 287:20201894. https://doi.org/10.1098/rspb.2020.1894
Kok EMA, Burant JB, Dekinga A et al (2019) Within-Individual Canalization Contributes to Age-Related Increases in Trait Repeatability: A Longitudinal Experiment in Red Knots. Am Nat 194:455–469. https://doi.org/10.1086/704593
Konishi K, Matsumura K, Sakuno W, Miyatake T (2020) Death feigning as an adaptive anti-predator behaviour: Further evidence for its evolution from artificial selection and natural populations. J Evol Biol 33:1120–1128. https://doi.org/10.1111/jeb.13641
López P, Martín J (2001) Fighting rules and rival recognition reduce costs of aggression in male lizards, Podarcis hispanica. Behav Ecol Sociobiol 49:111–116
Lorioux S, Lisse H, Lourdais O (2013) Dedicated mothers: predation risk and physical burden do not alter thermoregulatory behaviour of pregnant vipers. Anim Behav 86:401–408. https://doi.org/10.1016/j.anbehav.2013.05.031
Martín J, López P (2004) Iberian Rock Lizards (Lacerta monticola) Assess Short-Term Changes in Predation Risk Level When Deciding Refuge Use. J Comp Psychol 118:280–286. https://doi.org/10.1037/0735-7036.118.3.280
Martín J, López P, Polo V (2009) Temporal patterns of predation risk affect antipredator behaviour allocation by Iberian rock lizards. Anim Behav 77:1261–1266. https://doi.org/10.1016/j.anbehav.2009.02.004
Mattila ALK, Jiggins CD, Opedal ØH et al (2020) High evolutionary potential in the chemical defenses of an aposematic Heliconius butterfly. bioRxiv 2020.01.14.905950. https://doi.org/10.1101/2020.01.14.905950
McCabe K (2017) Within-Person Variability of Personality and Individual Differences. In: Zeigler-Hill V, Shackelford TK (eds) Encyclopedia of Personality and Individual Differences. Springer International Publishing, Cham, pp 1–4
Missoweit M, Engels S, Sauer KP (2007) Foraging ability in the scorpionfly Panorpa vulgaris: individual differences and heritability. Behav Ecol Sociobiol 61:487–492. https://doi.org/10.1007/s00265-006-0277-y
Montiglio P-O, Garant D, Pelletier F, Réale D (2012) Personality differences are related to long-term stress reactivity in a population of wild eastern chipmunks, Tamias striatus. Anim Behav 84:1071–1079. https://doi.org/10.1016/j.anbehav.2012.08.010
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nordberg EJ, Schwarzkopf L (2019) Predation risk is a function of alternative prey availability rather than predator abundance in a tropical savanna woodland ecosystem. Sci Rep 9:7718. https://doi.org/10.1038/s41598-019-44159-6
O’Hanlon JC, Rathnayake DN, Barry KL, Umbers KDL (2018) Post-attack defensive displays in three praying mantis species. Behav Ecol Sociobiol 72:176. https://doi.org/10.1007/s00265-018-2591-6
Osborn A, Briffa M (2017) Does repeatable behaviour in the laboratory represent behaviour under natural conditions? A formal comparison in sea anemones. Anim Behav 123:197–206. https://doi.org/10.1016/j.anbehav.2016.10.036
R Core Team (2020) A language and environment for statistical computing. Version 1.13. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Réale D, Festa-Bianchet M (2003) Predator-induced selection on temperament in bighorn ewes. Anim Behav 65:463–470. https://doi.org/10.1006/anbe.2003.2100
Rentz DC (1996) Grasshopper Country: The Abundant Orthopteroid Insects of Australia. UNSW Press, Sydney
Rosas JM, Todd TP, Bouton ME (2013) Context Change and Associative Learning. Wiley Interdiscip Rev Cogn Sci 4:237–244. https://doi.org/10.1002/wcs.1225
Ruxton GD, Allen WL, Sherratt TN, Speed MP (2019) Avoiding attack: the evolutionary ecology of crypsis, aposematism, and mimicry. Oxford University Press, Oxford
Sansom A, Lind J, Cresswell W (2009) Individual behavior and survival: the roles of predator avoidance, foraging success, and vigilance. Behav Ecol 20:1168–1174. https://doi.org/10.1093/beheco/arp110
Sargent TD (1990) Startle as an anti-predator mechanism, with special reference to the undenting moths, (Catocala). In: Evans D, Schmidt J (eds) Insect defenses: adaptive mechanisms and strategies of prey and predators. SUNY Press, Albany
Schlenoff DH (1985) The startle responses of blue jays to Catocala (Lepidoptera: Noctuidae) prey models. Anim Behav 33:1057–1067. https://doi.org/10.1016/S0003-3472(85)80164-0
Schmidt AKD, Römer H, Riede K (2013) Spectral niche segregation and community organization in a tropical cricket assemblage. Behav Ecol 24:470–480
Schmitz OJ, Beckerman AP, O’Brien KM (1997) Behaviorally Mediated Trophic Cascades: Effects of Predation Risk on Food Web Interactions. Ecology 78:1388–1399. https://doi.org/10.1890/0012-9658(1997)078[1388:BMTCEO]2.0.CO;2
Segovia JMG, Murayama GP, Willemart RH (2019) Sexual differences in weaponry and defensive behavior in a neotropical harvestman. Curr Zool 65:553–558. https://doi.org/10.1093/cz/zoy073
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Tanis BP, Bott B, Gaston BJ (2018) Sex-based differences in anti-predator response of crickets to chemical cues of a mammalian predator. PeerJ. https://doi.org/10.7717/peerj.4923
Turney S, Godin J-G (2014) To forage or hide? Threat-sensitive foraging behaviour in wild, non-reproductive passerine birds. Curr Zool 60:719–728. https://doi.org/10.1093/czoolo/60.6.719
Umbers KDL, De Bona S, White TE et al (2017) Deimatism: a neglected component of antipredator defence. Biol Lett 13:20160936. https://doi.org/10.1098/rsbl.2016.0936
Umbers KDL, Mappes J (2015) Postattack deimatic display in the mountain katydid, Acripeza reticulata. Anim Behav 100:68–73. https://doi.org/10.1016/j.anbehav.2014.11.009
Umbers KDL, White TE, De Bona S et al (2019) The protective value of a defensive display varies with the experience of wild predators. Sci Rep 9:463. https://doi.org/10.1038/s41598-018-36995-9
Vidal-García M, O’Hanlon J, Svenson GJ, Umbers KDL (2020) The evolution of startle displays: a case study in praying mantises. Proc R Soc B 287:20201016
Walters ET (1994) Injury-Related Behavior and Neuronal Plasticity: an Evolutionary Perspective on Sensitization, Hyperalgesia, and Analgesia. In: Bradley RJ, Harris RA (eds) International Review of Neurobiology. Academic Press, pp 325–427
Watkins AJ, Goldstein DA, Lee LC et al (2010) Lobster Attack Induces Sensitization in the Sea Hare, Aplysia californica. J Neurosci 30:11028–11031. https://doi.org/10.1523/JNEUROSCI.1317-10.2010
Wells MJ (1968) Sensitization and the Evolution of Associative Learning. In: Salánki J (ed) Neurobiology of Invertebrates: Proceedings of the Symposium Held at the Biological Research Institute of the Hungarian Academy of Sciences (Tihany) September 4–7, 1967. Springer US, Boston, pp 391–411
Wickham H (2020) reshape2: Flexibly Reshape Data: A Reboot of the Reshape Package. Version 1.4.4URL https://github.com/hadley/reshape
Wickham H (2019) stringr: Simple, Consistent Wrappers for Common String Operations. Version 1.4.0URL http://stringr.tidyverse.org
Wickham H, François R, Henry L, Müller K (2020) dplyr: A Grammar of Data Manipulation. Version 1.0.2URL https://dplyr.tidyverse.org
Worthington AM, Swallow JG (2010) Gender differences in survival and antipredatory behavior in stalk-eyed flies. Behav Ecol 21:759–766. https://doi.org/10.1093/beheco/arq050
Ydenberg RC, Dill LM (1986) The Economics of Fleeing from Predators. In: Rosenblatt JS, Beer C, Busnel M-C, Slater PJB (eds) Advances in the Study of Behavior. Academic Press, pp 229–249
York CA, Bartol IK (2016) Anti-predator behavior of squid throughout ontogeny. J Exp Mar Biol Ecol 480:26–35. https://doi.org/10.1016/j.jembe.2016.03.011
Acknowledgements
Thanks to Marie Herberstein for helpful discussion in designing experiments, Brendan Baker for scoring videos, Redbank Ski Lodge for providing discounted accommodation and Thredbo Sports for lift passes. This study was funded by the Hermon Slade Foundation, Project: HSF14/3 and Western Sydney University.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the Hermon Slade Foundation, Project: HSF14/3, Western Sydney University.
Author information
Authors and Affiliations
Contributions
The study conception and design, material preparation and data collection were carried out by Sebastiano De Bona and Kate DL Umbers. Analysis was carried out by Sebastiano De Bona, Kate DL Umbers and Faelan Mourmourakis. The manuscript was written by Faelan Mourmourakis with input from all authors who have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
All authors certify that they have no conflicts of interest or relevant financial interests to declare that are relevant to the content of this article.
Additional information
Communicated by K. Shaw.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mourmourakis, F., De Bona, S. & Umbers, K.D. Increasing intensity of deimatic behaviour in response to repeated simulated attacks: a case study on the mountain katydid (Acripeza reticulata). Behav Ecol Sociobiol 76, 118 (2022). https://doi.org/10.1007/s00265-022-03226-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03226-1